J. Northw. Atl. Fish. Sci., Vol. 55: 11-29
Gary A. Nelson*1, Kara L. Duprey2, and Scott P. Elzey2 1
1Massachusetts Division of Marine Fisheries
92 Fort Avenue, Salem MA, 01970
(gary.nelson@mass.gov)
2Massachusetts Division of Marine Fisheries
30 Emerson Avenue, Gloucester, MA 01930
(kara.l.duprey@mass.gov; scott.elzey@mass.gov)
*Corresponding Author
Nelson, G.A., Duprey, K.L., and Elzey, S.P. 2024. Aspects of the Population Dynamics and Biology of the Daubed Shanny (Leptoclinus maculatus) from the Gulf of Maine. J. Northw. Atl. Fish. Sci., 55: 11–29. https://doi.org/10.2960/J.v55.m747
Abstract
The daubed shanny (Leptoclinus maculatus) is an Arctic-boreal fish species with a circumpolar distribution and whose southernmost extent of its range in the northwest Atlantic is the Gulf of Maine. Because life history characteristics of fishes often vary along latitudinal gradients, the daubed shanny population in the Gulf of Maine may exhibit different biological characteristics and population dynamics than the Arctic populations from which most information about the species comes. To improve our knowledge, this study was undertaken to document trends in temporal abundance, spatial abundance, and depth and temperature ranges based on historical trawl surveys, and to evaluate sex-specific differences in size, weight and age of individuals captured in the Gulf of Maine. The species was distributed throughout the western Gulf of Maine, primarily at depths from 30 to 120 m in spring and in waters ≥82 m in fall and was associated with the near-lowest temperatures available in the survey regions. Most daubed shanny were ≥8–9 cm total length in spring, but small fish (7–11 cm total length) dominated catches in fall, possibly representing pelagic post-larvae settling to the benthos. The population abundance of daubed shanny fluctuated widely since 1963 but appeared to collapse after 2009 in concert with warming temperatures and declines in Calanus copepod abundance in the Gulf of Maine. Female daubed shanny were larger and heavier than males, and both sexes reached a maximum age of six years. Compared to published data, daubed shanny in the Gulf of Maine has a shorter life span, grows faster and likely experiences higher natural mortality than the Svalbard, Norway, population above the Arctic circle.
Keywords: Abundance, Gulf of Maine, Leptoclinus maculatus, population biology
PDF Supplementary Materials
Download Citation Data

Citation to clipboard
 Reference management software (Endnote, Mendeley, RefWords, Zotero & most other reference management software)
Reference management software (Endnote, Mendeley, RefWords, Zotero & most other reference management software)
LaTex, BibDesk & other specific software
Introduction
The daubed shanny (Leptoclinus maculatus) is an Arctic-boreal benthic species of the family Stichaeidae that has a circum-polar Arctic distribution between latitudes 43°N and 79°N (Mecklenburg et al., 2011; Meyer Ottesen et al., 2011). In the North Atlantic, this species ranges from the Arctic south to Norway and Sweden on the eastern side, and to southern Gulf of Maine, USA, on the western side (Scott and Scott, 1988; Collette, 2002; Meyer Ottesen et al., 2011). Throughout its range, daubed shanny is a prey species for marine fishes (Bowman et al., 2000; Hovde et al>., 2002), sea birds (Bryant et al., 1999; Jones et al., 2002; Elliot et al., 2008) and marine mammals (Labansen et al., 2007; Lesage et al., 2020). Species of the Stichaeidae family are considered intermediate links between zooplankton and higher trophic levels (Murzina et al., 2012).
Most knowledge of the life history of daubed shanny comes from studies in the Barents Sea and around Svalbard, Norway in waters north of 75°N. In those areas, the species is typically found at depths between 2 and 475 m with temperatures between -1.6 and 2.0°C (Andriyashev, 1954; Byrkjedal and Høines, 2007). Daubed shanny reaches a maximum of about 200 mm total length (TL), but most individuals are <170 mm or so (Andriyashev, 1954). Females and males mature between 125- and 130-mm standard length at about 7 and 6 years, respectively, and males grow faster and tend to be larger than females (Meyer Ottesen et al>., 2014). Adults spawn in the winter, probably in shallow water, and females produce few (<1000), large (0.7–1.7 mm) eggs (Murzina et al., 2012; Meyer Ottesen et al., 2014, 2018). Daubed shanny likely exhibits parental care where females guard eggs and males defend territories in soft bottom areas like other Stichaeidae (Meyer Ottesen et al., 2011), and has been observed residing in burrows (Meyer Ottesen et al., 2011). It is unknown if daubed shanny larvae have a yolk-sac stage, but the post-larvae remain in the pelagic environs feeding on Calanus copepods and storing lipids in a specialized sac (as a winter food source) for 2–3 years and then settle to the benthic habitat when they reach about 80 mm caudal length (Meyer Ottesen et al., 2011; Pekkoeva et al., 2023).
In the Gulf of Maine, the southernmost extent of its range, almost nothing is known about daubed shanny. Information on biology cited in Bigelow and Schoeder’s Fishes of the Gulf of Maine (Collette, 2002) comes from studies conducted in its northern range with the exceptions of Tyler (1971) who caught daubed shanny during a community structure study in a Maine estuary, and Bowman et al. (2000) who listed the species as a diet item of other Gulf of Maine fishes. Given that life history characteristics of fishes with wide latitudinal ranges often vary along latitudinal gradients (Legget and Carscadden, 1978; Shepherd and Grimes, 1983; Trip et al., 2014; Riesch et al., 2018), the daubed shanny population in the Gulf of Maine may exhibit different biological characteristics and population dynamics than the northern populations. Therefore, to improve our knowledge, this study was undertaken to document trends in temporal and spatial abundance, depth and temperature ranges, and size structure of daubed shanny based on historical trawl survey data, and to evaluate sex-specific differences in size, weight and age of individuals captured in the Gulf of Maine.
Materials and Methods
Historical Data
Fig. 1
Data used to analyze trends in historical abundance, spatial distribution, depth and temperature, and size composition came from the Northeast Fisheries Science Center (NEFSC), Maine Department of Marine Resources (MEDMR) and Massachusetts Division of Marine Fisheries (MADMF) spring and fall bottom trawl surveys in the Gulf of Maine (GoM; Fig. 1). The details of each survey are described below.
The NEFSC survey was initially based on a stratified random sampling design for continental shelf water >27 m in depth, partitioned into strata of unequal area based on depth and geographic location (Azarovitz, 1981). In the Gulf of Maine, the survey occurs in both US and Canada territorial seas. From 1963 to 1967, sampling was conducted in fall (September–November). A spring (March–May) survey was added in 1968. Sampling effort was expanded in 1972 to include the inshore waters, but effort did not begin in the Gulf of Maine until 1979. Sampling stations were randomly selected within each stratum and allocated among strata in proportion to stratum area. From 1963–2008, a standard #36 or #41 Yankee bottom trawl with a 1.25 cm stretched mesh cod-end liner was towed by primarily the R/V Albatross IV (the R/V Delaware was used on occasion to supplement sampling) at each station at approximately 3.5 knots for 30 minutes; depth (at the start and end of each tow) and temperature (after arriving on station) were also recorded (Azarovitz, 1981). In 2009, a new, much larger modern vessel, R/V Henry B. Bigelow, replaced the R/V> Albatross IV. Major changes to the fishing methods and survey design were also made. A four-seam bottom trawl replaced the #36 Yankee trawl and tow duration was reduced to 20 minutes and, due to the vessel size, some inshore strata were eliminated. Relative catch efficiency studies of the R/V Henry B. Bigelow and R/V Albatross IV for many fish species have been conducted (Miller et al., MS 2010; Miller, 2013), but conversion factors are not available for the daubed shanny; therefore, no adjustments to catch after 2008 were made. Data from the inshore (strata: 57–90) and offshore (strata: 24, 26–40) regions of the Gulf of Maine (see Supplemental Document Appendix SA for spatial maps of strata) from 1963–2022 were used in the analyses. Spring and fall surveys were not conducted in 2020 due to COVID restrictions.
The MADMF survey began in 1978 in Massachusetts territorial waters and was conducted in spring (May) and fall (September)(Howe et al., MS 2002). The survey used a stratified random sampling design with strata defined by region and depth. Regions were stratified into depth strata: ≤9 m, 10–18 m, 19–27 m, 28–36 m, 37–55 m, and >55 m. Sampling sites were proportionally allocated to each stratum based on stratum area. A 20-minute tow (vessel speed is 2.5 knots) was made at each station with a two-seam 3/4 Whiting trawl that contains a 6.3 mm-mesh cod-end liner. At each station, starting depth and ending depth were recorded for a tow (only starting depth was recorded from 1978–1980), and a marine hydrographic instrument was used to record bottom water temperature at the end of each tow. The survey was initially conducted with the R/V Wilbur in 1978 and 1979, but later switched to the NEFSC vessel R/V Gloria Michelle in 1980. Only data from strata defined as occurring in the Gulf of Maine (strata: 25–36) were used in analyses (see Supplemental Document Appendix SB for a spatial map of strata). The spring and fall surveys were not conducted in 2020 due to COVID restrictions.
The MEDMR inshore trawl survey began in 2000 and mainly covered New Hampshire and Maine waters shallower than 120 meters. In 2003, strata were expanded to deeper waters. The MEDMR survey used a stratified random design. The areas sampled included four depth strata: 9–36 m, 37–64 m, 65–100 m, and >100 m out to approximately the 12-mile limit, and five longitudinal regions based on oceanographic, geologic, and biological features (Shermanet al., MS 2005). Number of tows was proportionally allocated to each stratum based on area. Two virtually identical commercial fishing vessels, the F/V Tara Lynn and F/V Robert Michael, were used for this survey. Both vessels were Down East 54’s constructed of a combination of solid and sandwich fiberglass. Until spring of 2004, the two vessels alternated between spring and fall surveys. Since spring of 2004, the survey has been conducted solely by the F/V Robert Michael. The net used was a modified version of a shrimp net design for Maine waters (see Sherman et al., MS 2005 for specifications) constructed of 5 cm polyethylene mesh and a 2.54 cm cod-end mesh liner and was towed for 20 minutes at each station. Depth was recorded at the start and end of each tow, and temperature was recorded at the end of each tow. We used data from all strata but restricted our analyses to years 2003–2023 to maintain design consistency across years (see Supplemental Document Appendix SC for a spatial map of strata). The spring survey was not conducted in 2020 due to COVID restrictions.
During all surveys, fish captured in the otter trawls were brought onboard, sorted to species, counted, weighed collectively to the nearest 0.1 kg and measured individually to the nearest cm (daubed shanny TL was measured). If tow catch of a fish species was deemed large, a random subsample was taken for length measurements and expanded to the total catch during data processing. Errors in tow data were identified mainly via different statistical summaries and plots; if found, data were eliminated from analyses.
Abundance
Relative abundance indices for spring and fall were computed as the stratified mean number of daubed shanny per standardized tow (Fogarty, 1985; Thompson, 2002):
where Ak is the area of stratum k and is the mean number per haul in stratum k. The standard error was calculated as
where is the variance of the mean number per haul in stratum k defined as
varY-k=sk2nk
where sk2 is the sample variance and n kis the number of tows in stratum k. Standardized tow data deemed acceptable were extracted by using gear/tow condition codes for each survey (Supplemental Document Table SA). Catch data (numbers) were transformed using loge(y+1) prior to calculation to stabilize variance and reduce the influence of sampling variability between tows (Fogarty, 1985; Sokal and Rohlf, 1995). The R package survey (Lumley, 2023) was used for all computations. Function svydesign was used to define the survey design (fpc = stratum area) and then function svymean was used to calculate means and standard errors. For the NEFSC survey, indices were calculated separately for the inshore and offshore strata regions.
To determine if trends in seasonal relative abundances were similar within and among surveys, the Spearman rank correlation test (Sokal and Rohlf, 1995) was applied to pairings of indices that were truncated to match the span of the shortest time series in the comparison.
Depth, Temperature and Size Distributions
Abundance-weighted mean depth (m) and temperature (°C) were calculated by season to examine patterns in spatial and temporal distributions of catches. Data from all years were combined for these analyses to increase sample sizes of positive tows and individuals for analyses. The abundance-weighted mean for depth or temperature was calculated as
and its standard deviation
SD=∑i=1P'wi(xi-X-)2((P'-1)∙∑i=1P'wi)/P'
where wi is the number of fish in non-zero tow i, P′ is the number of non-zero weights, and xi is either depth or temperature at tow i. For all analyses, the mean depth of a tow was used. All calculations were performed by using functions svymean, svyvar and svyquantile.
To determine if the depth and temperature patterns observed reflected strong affinities of daubed shanny for specific ranges of depth and temperature, the method of Perry and Smith (1994) was used to test if the depth and temperature associated with fish abundance was significantly different from the ranges of environmental conditions measured by each survey. Briefly, empirical cumulative distribution functions (CDF) of a habitat variable for the survey and for fish were constructed and the Kolmogorov-Smirnov maximum absolute vertical distance (Ds) statistic (Siegel, 1956) between CDFs was calculated and then compared to a null distribution of the test statistic created via randomization. The empirical cumulative distribution function for a habitat variable of the complete survey was constructed by
where the I(xki) is an indicator function of the form
and t represents an index, ranging from the lowest to the highest value at a step size appropriate for the desired resolution (Perry and Smith, 1994). The habitat CDF for a species was constructed by
where yk,i is the ith tow in stratum k. The Ds test statistic was calculated as
The null distribution of the test statistic was constructed by randomizing pairings of
and xk,i over all k and i and then calculating the test statistic (D) for the new pairs. The xk,i for the pairings was obtained by sampling with replacement the observed xk,i with probability Wknk. This procedure was repeated 2000 times and the significance level determined by dividing the number of times D≥ Ds by 2000. The method was applied to catch data grouped into seasons and regions.
Regional and seasonal changes in size structure were investigated by comparing summary statistics and seasonal length frequencies (computed as proportions in 1-cm length intervals) among surveys. Due to the variable nature of catches, data from all years were combined for most analyses. All summary statistics and proportions were calculated assuming a random cluster design (Nelson, 2014). Bootstrapping was used to estimate standard errors of proportions-at-length.
Biology
Daubed shanny were collected opportunistically for biology and ageing information and subsampled non-proportionally from three (stations 24, 26 and 27) out of eight positive tows located in Cape Cod Bay and off Gloucester, Massachusetts in spring of 1990 during the MADMF trawl survey (Fig. 1). Subsampled fish (n = 101) were frozen at sea. In the laboratory, individuals were thawed, and total length (TL ±1 mm) and total weight (TW ±0.001 g, measured after each fish was blotted dry) were recorded for all individuals. Sex determination was accomplished by macroscopically and microscopically inspecting gonads.
Because fish were not proportionally subsampled from hauls, data were weighted before analyses. A weighted correction applied to individual data from each haul in subsequent analyses was based on the inverse sampling fraction:
wh=Nhnh
where wh is the weight for haul h,nh is the number of fish subsampled and Nh is the total number caught in haul h. The haul weights derived for stations 24, 26, and 27 were 2.2667 (34/15), 2.0888(94/45) and 1.7073 (70/41), respectively.
Sex-specific differences in length and weight were explored via summary statistics calculated by using function svymean, and by using the design-based two-sample Kruskall-Wallis test (function svyranktest; Lumley and Scott, 2013) to test for significant differences in median values. Differences in total weight versus total length relationships were also explored. One-way analysis of covariance (Sokal and Rohlf, 1995), conducted by using function svyglm, was used to test for differences in intercepts and regression slope parameters between sexes. Total length and total weight were log10 transformed prior to estimation. The full model fitted was
where log10TL was treated as a continuous predictor, sex as a factor, and an interaction term between the two predictors was included. The anova.svyglm function was used to test whether the sequential addition of a predictor significantly contributed to a reduction in overall variance by using the Rao-Scott Likelihood Ratio Test (Lumley and Scott, 2014), and diagnostic plots (standardized residuals-fitted values, qq-residuals and residuals-leverage) were examined for potential violations of model assumptions.
Sagittal otoliths were used for ageing. Sagittae were removed, cleaned of soft tissue and stored dry in coin envelopes, which after 34 years, were free of any decomposition. One otolith was randomly picked from an individual and cut in half close to the core by using an Isomet saw. Both halves were mounted core-side up on slides with Flo-Texx and viewed in mineral oil under reflected light on a computer monitor using Image Pro 10 connected to an Infinity Lumenera 3 camera and Optem Zoom 125 lens tube. Annuli were identified following standard protocols developed for other northwest Atlantic fishes (Elzey et al., MS 2015). Age determinations were made by the latter two authors who are experienced age readers. If age readings disagreed, a final age was reached via consensus. Daubed shanny spawn in winter (Pekkoeva, et al., 2018); therefore, a January 1 birthdate was assumed. Although not processed until 2024, there was no apparent degradation of the otoliths that may have comprised the accuracy of age determination.
Sex-specific differences in age composition were explored as described above for length and weight. Due to the limited sample sizes, growth curves were not fitted to length and age data; rather, data were summarized to compare mean size- and weight-at-age between sexes and to compare results to previously published growth information for the Svalbard population of daubed shanny (Meyer-Ottesen et al., 2014). The caudal-length (Meyer Ottesen et al., 2011) sizes from Meyer-Ottesen et al. (2014) were converted to total length by using a ratio of 1.08 derived from photographs of individuals in Pekkoeva et al. (2018) and Meyer Ottesen et al. (2018).
Results
Abundance
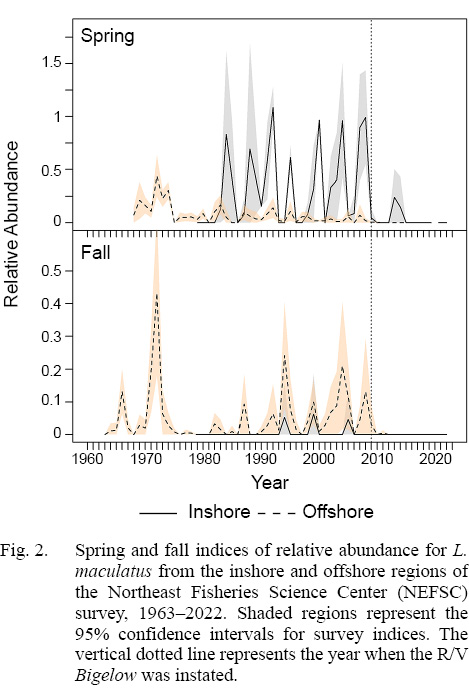
Fig. 2
Number-per-tow indices for daubed shanny fluctuated markedly from 1963–2023 in NEFSC regions. In the inshore region prior to 2009, spring and fall indices showed little trend as means varied widely over time due to low number of tows made annually and low positive tows (Fig. 2; see Supplemental Document Tables SB and SC for statistics). Although daubed shanny were sporadically caught in the offshore region, some weak trends in relative abundance were observed. Stratified means were highest in the late 1960s to early 1970s in spring and fall, but then declined to lower levels in the mid-1970s (Fig. 2). Starting in the mid-1990s, spring indices declined further and remained low through 2008, but fall indices increased during the same period. After the switch to the R/V Bigelow in 2009, daubed shanny were rarely caught in any region or season (Fig. 2). Correlations among inshore and offshore indices were only significant in fall (ρ = 0.47, p = 0.008, n = 30); however, this result should be viewed with caution given that inshore means were greater than zero in only 3 out of 30 years (Fig. 2).
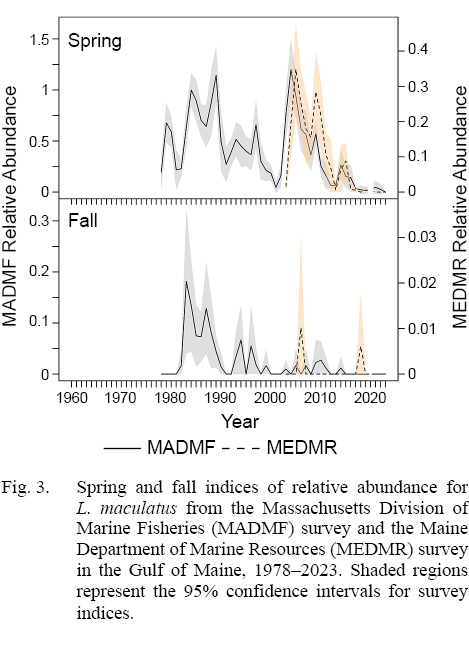
Fig. 3
In Massachusetts waters, mean numbers-per-tow were high in the 1980s in spring and fall but declined in the 1990s (Fig. 3; see Supplemental Document Table SD for statistics). Spring relative abundance peaked again in 2004, gradually declined through 2013, increased slightly in 2014, but then declined to near zero starting in 2017. No fish were caught in 2023. There was little trend in the fall index except daubed shanny were not captured after 2014. Daubed shanny were typically caught in higher numbers in spring than in fall (Fig. 3). The spring and fall indices were significantly correlated (ρ = 0.62, p = 0.000, n = 45).
Similar temporal trends and seasonal patterns in relative abundance were observed in the MEDMR survey compared to the MADMF survey (Fig. 3; see Supplemental Document Table SE for statistics). Mean numbers-per-tow were highest in spring. Spring indices peaked in 2005 and 2009, but then steadily declined to zero or near zero levels in 2016. No fish were captured after 2020 (Fig. 3). Trends in the spring and fall indices were not significantly correlated (ρ = 0.01, p = 0.973, n =2 0).
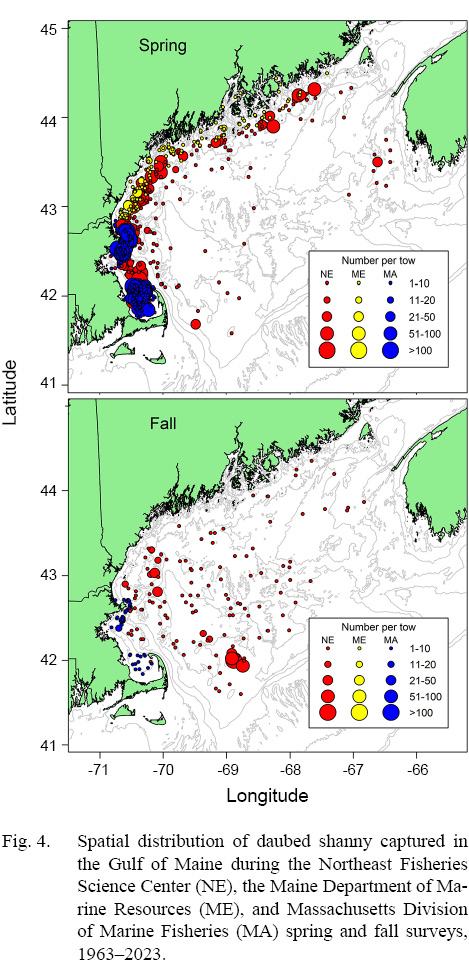
Fig. 4
Results of the Spearman rank correlation tests indicated that, among all survey indices, the only significant pairings were between the MEDMR spring index and the MADMF spring and fall indices (MEDMR spring versus MADMF spring: ρ = 0.85, p = 0.000, n = 20; MEDMR spring versus MADMF fall: ρ = 0.60, p = 0.005, n = 20).
Depth, Temperature and Size Distributions
Changes in depth, temperature and size distributions were evident across seasons.
Spring. Catches of daubed shanny were primarily distributed in the western Gulf of Maine from Massachusetts to just north of Bar Harbour, Maine (Fig. 4). Fish catches occurred in waters between 11 and 79 m (D-: 49.0 m; SD: 13.63 m) with bottom temperatures between 3.0 and 8.9 °C (: 4.0 °C; SD: 0.60 °C) in the NEFSC inshore region, and at depths between 33 and 233 m (: 84.6 m; SD: 22.91 m) with bottom temperatures between 2.2 and 8.3°C ( 4.4°C; SD range: 0.86°C) in the NEFSC offshore region (Table 1). Most daubed shanny (95%) were captured in depths ≥31 m and ≤130 m in the inshore and offshore regions, respectively (Table 1). Comparisons between the NEFSC fish and survey CDFs indicated that fish catches were significantly associated with the deeper waters and lower temperatures of the inshore region, and shallower depths and lower temperatures of the offshore region (Fig. 5). The sizes of fish ranged between 5 and 22 cm TL, but most (97.5%) individuals were ≥8–9 cm TL, and few fish >16 cm TL were captured (Fig. 6). Mean lengths for all years combined were similar between the inshore and offshore regions, but the largest individuals (>16 cm TL) were captured in the offshore region (Fig. 6).
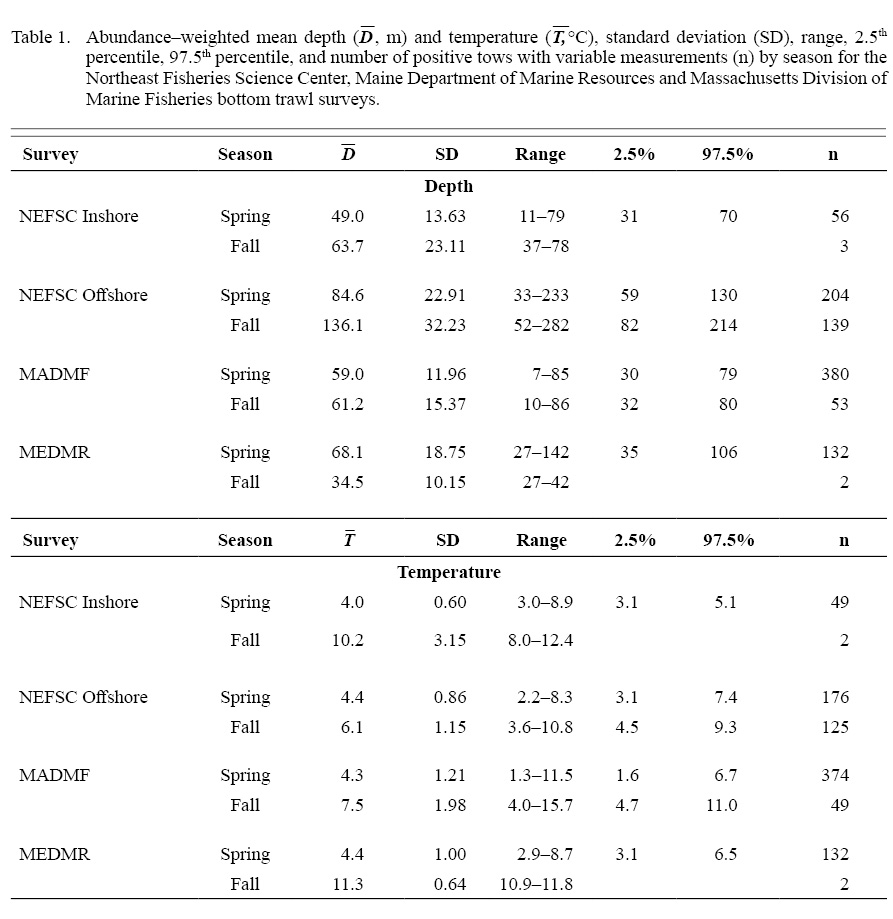
Table 1
In the MADMF survey, daubed shanny catches were concentrated in Cape Cod Bay and areas surrounding Gloucester, Massachusetts (Fig. 4) at depths between 7 and 85 m (: 59.0 m; SD: 11.96 m) with bottom temperatures between 1.3 and 11.5°C (: 4.3°C; SD: 1.21°C ). Most catches (97.5%) occurred in waters ≥30 m (Table 1). Catches were significantly associated with the deeper waters and lower temperatures of the sampled survey area (Fig. 5). The sizes of captured fish ranged between 4 and 22 cm TL, but most individuals (97.5%) were ≥8 cm TL, and few fish were >17 cm TL (Fig. 7). Fish were slightly larger than those caught in the NEFSC survey (Figs. 6 and 7).
Daubed shanny captured in the MEDMR survey were distributed and concentrated in similar areas as the NEFSC survey (Fig. 4). Catches occurred in waters between 27 and 142 m (: 68.1 m; SD: 18.75 m) with bottom temperatures between 2.9 and 8.7°C (: 4.4°C ; SD: 1.0°C). Most catches were taken at depths ≥35 m and were significantly associated with the deeper waters and lower temperatures of the survey area (Fig. 5). Sizes of daubed shanny ranged between 8 and 22 cm TL, but most individuals (97.5%) were ≥9 cm TL, and few fish >16 cm TL were captured (Fig. 7). Fish were similar in size compared to individuals captured in the MADMF survey (Fig. 7).
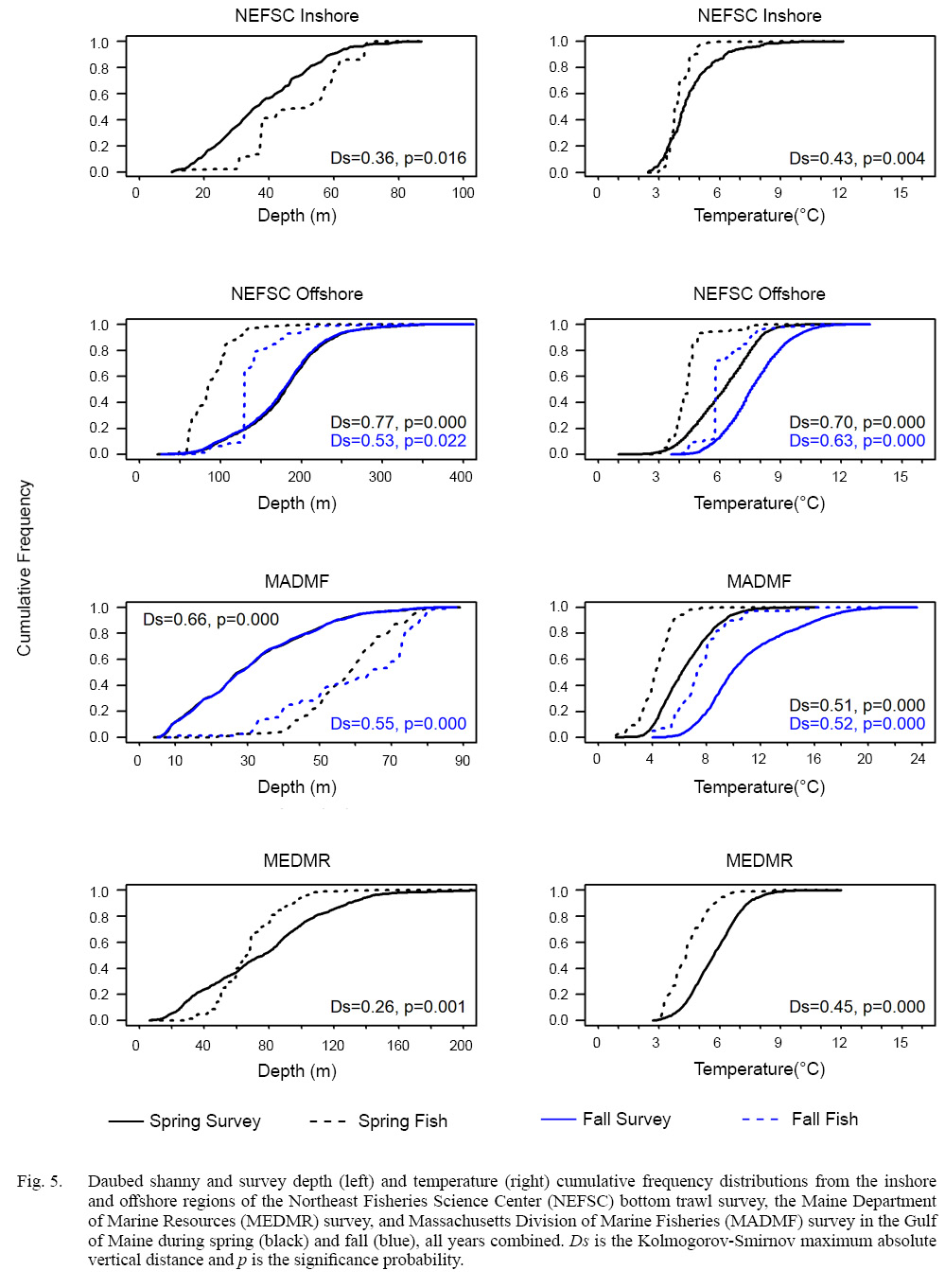
Fig. 5
Among-survey comparisons revealed that daubed shanny were caught in relatively consistent temperatures in spring regardless of fish depth (Table 1), suggesting the species either experienced a well-mixed thermal environment or it preferred specific temperature ranges. Comparison of fish abundance-weighted mean temperatures and survey mean temperatures (with associated standard deviations), calculated for 10-m depth intervals, indicated that daubed shanny likely selected specific temperatures because fish mean temperatures for the shallower, warmer nearshore surveys (NEFSC inshore region, MADMF and MEDMR) were in the lower range of temperatures measured by the surveys and were generally consistent across depths (Fig. 8)(see Supplemental Document Tables SF and SG for statistics).
Fall. Catches of daubed shanny were low compared to spring and were primarily distributed throughout Gulf of Maine away from nearshore areas (Fig. 4). In the offshore region of the NEFSC survey, catches occurred in deeper waters between 52 and 282 m (: 136.1 m; SD: 32.23 m) with bottom temperatures between 3.6 and 10.8C T-: 6.1°C; SD: 1.15°C), but mostly in depths ≥82 m (Table 1). Although the distribution shifted to deeper waters, daubed shanny catches were still significantly associated with the shallower waters and lower temperatures of the survey area (Fig. 5), and fish selected the lower range of temperatures across depths as in spring (Fig. 8; see Supplemental Document Table SH for statistics). Compared to spring, fall catches were dominated by small individuals 7–11 cm TL (L-: 8.2 cm TL, mode: 8 cm TL), and few fish >11 cm TL were caught (Fig. 6).
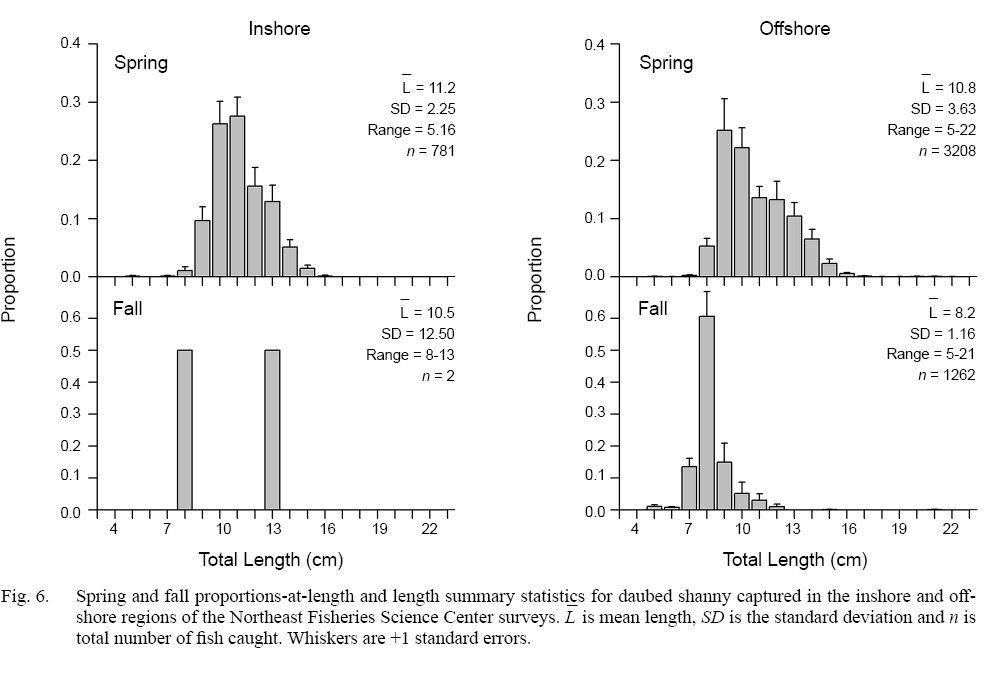
Fig. 6
In the MADMF survey, low catches were distributed similarly as in spring (Fig. 4) and were significantly associated with the deeper waters and lower temperatures of the sampled survey area (Fig. 5). The size composition of the MADMF survey in fall was similar to the size composition in spring (Fig. 7). Few positive tows and very low numbers of daubed shanny caught in the NEFSC inshore region and MEDMR survey precluded analyses of depth and temperature data.
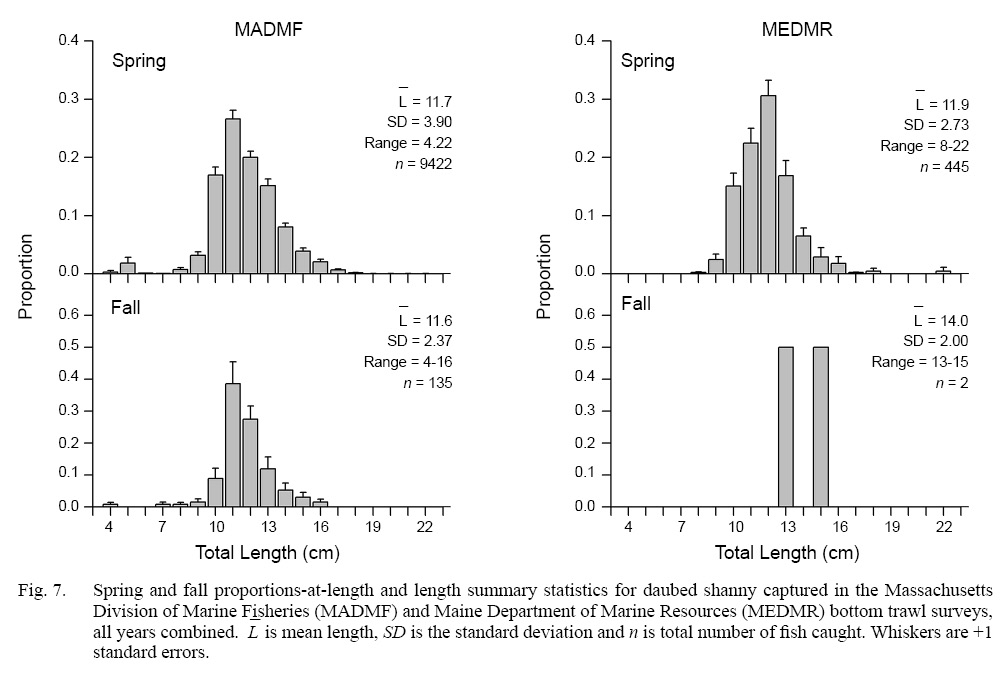
Fig. 7
Biology
The 101 fish subsample (out of 297 fish caught in the entire 1990 survey) consisted of 56 females, 41 males and 4 unsexed individuals ranging in size from 100 to 167 mm TL and were similar to the range of sizes (~92–167 mm TL) captured by Meyer Ottensen et al. (2014). Sex composition of the subsamples was consistent across tows: female proportions ranged from 54 to 60%. The final weighted sex composition was 58% female and 42% male. Gulf of Maine females were significantly larger (L- = 144.3 mm, SD = 14.92 mm, range: 103–167 mm, n = 56) and heavier (W- = 9.8 g, SD = 3.22 g, range: 3.2–17.2 g, n = 56) than males ( =132.6 mm, SD = 9.75 mm, range:113–152 mm, n = 41; W- = 7.7 g, SD = 2.15 g, range: 3.3–12.0 g, n = 40) (Fig. 9).
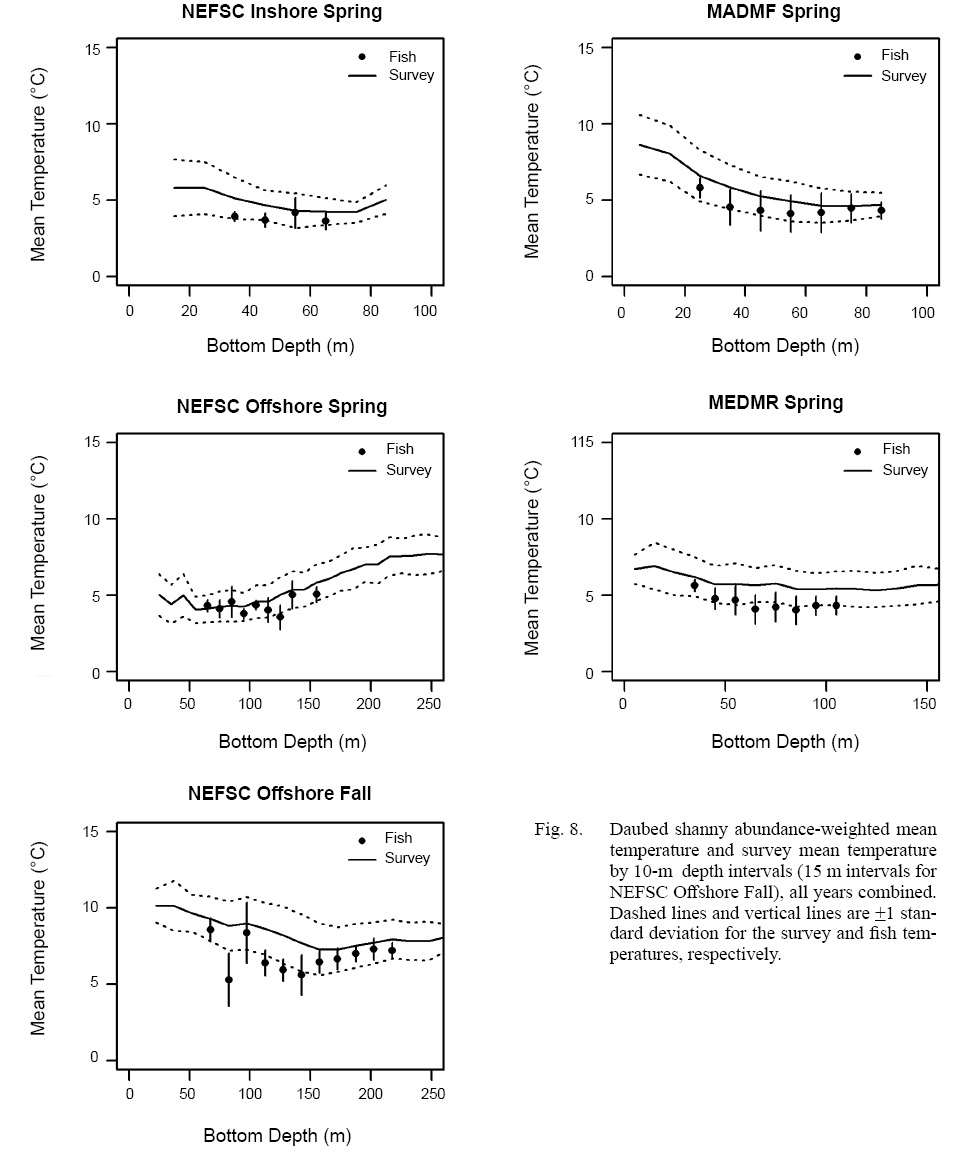
Fig. 8
Significant intercept, slope, sex factor and interaction term indicated the total-length on total-weight relationships were different between sexes (Table 2). The regression slope for males was steeper (3.89), but less precise, than the slope (3.34) for females, indicating the former gained more weight per unit increase in length than the latter. Although diagnostics plots showed that the model described the length-weight relationships well, the smaller sample size for males may not have captured the relationship adequately. Therefore, a combined sex model was fitted to provide an overall weight-length relationship for the species that may be impacted less by sample size (Table 2).
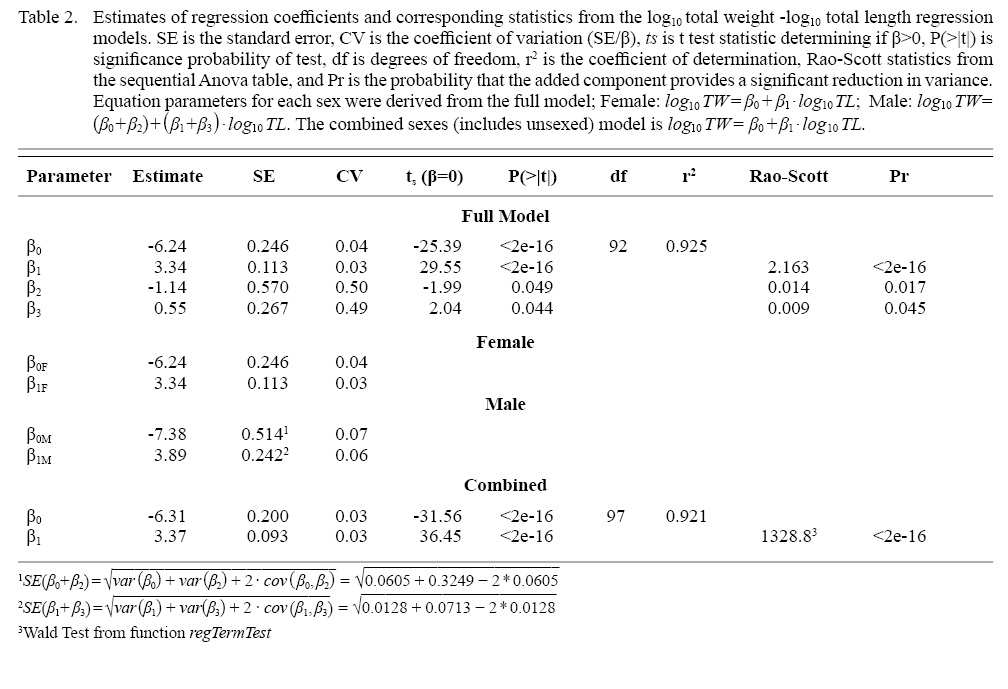
Table 2
Ages of daubed shanny ranged from 1 to 6 years. The majority (female: 95%; male: 92%) were ≥3 years; there was no difference in age distributions between sexes (Fig. 9). Comparison of mean length- and mean weight-at-age for ages 3 and 4 (ages with largest sample size) indicated that females were larger and heavier than males of the same age (Table 3), implying females grow faster than males in the Gulf of Maine. Additionally, mean length-at-age for ages 3 and 4 are much larger than growth curve predictions of mean lengths at ages 3 and 4 for females (~71 and ~104.9 mm TL, respectively) and males (~100 and ~112 mm TL, respectively) in Meyer Ottesen et al. (2014), suggesting Gulf of Maine fish grow faster than the Svalbard population as well.
Discussion
Abundance
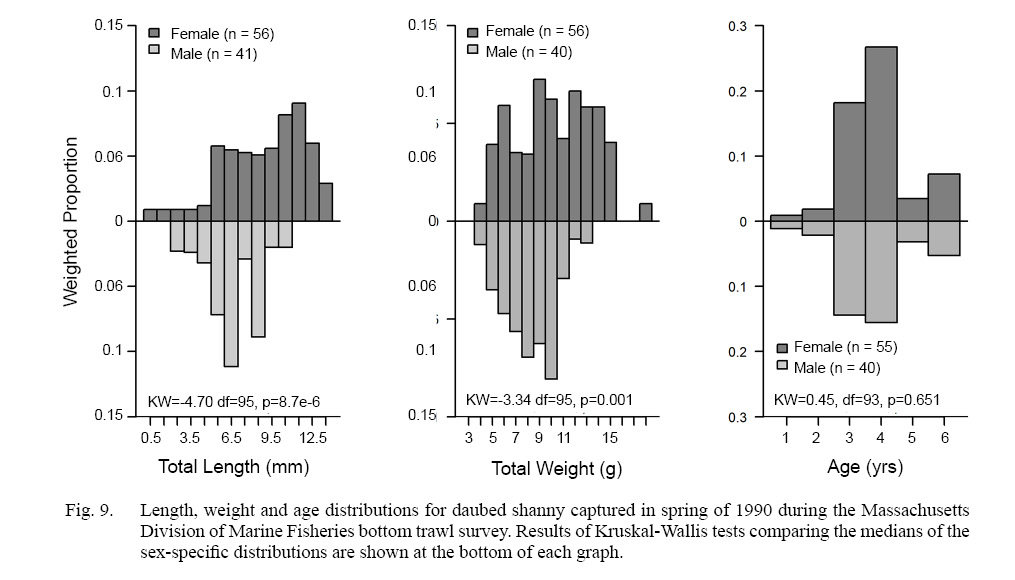
Fig. 9
The historical status of daubed shanny population abundance, as measured by three bottom trawl surveys, was difficult to determine because of disparate patterns in relative abundance indices among surveys and seasons. The highest catches and encounters (positive tows) occurred in spring in all surveys, suggesting spring indices may reflect population trends best. However, the spring regional indices from the NEFSC survey are likely unreliable measures of abundance because of the sporadic encounters of daubed shanny that created quite variable fluctuations in relative abundance and little correlation among regions. The variable nature of encounters in this survey may be due to the species possible preference for mud/silt habitats (Meyer Ottesen et al., 2014), the nets used during the surveys, and changes in net design, vessel usage and door design over time (Sissenwine and Bowman, 1978; Azarovitz, 1981; NEFSC, MS 1997). With the instatement of the R/V Bigelow in 2009, it appears the NEFSC survey will have little ability to monitor daubed shanny in the Gulf of Maine in the future.
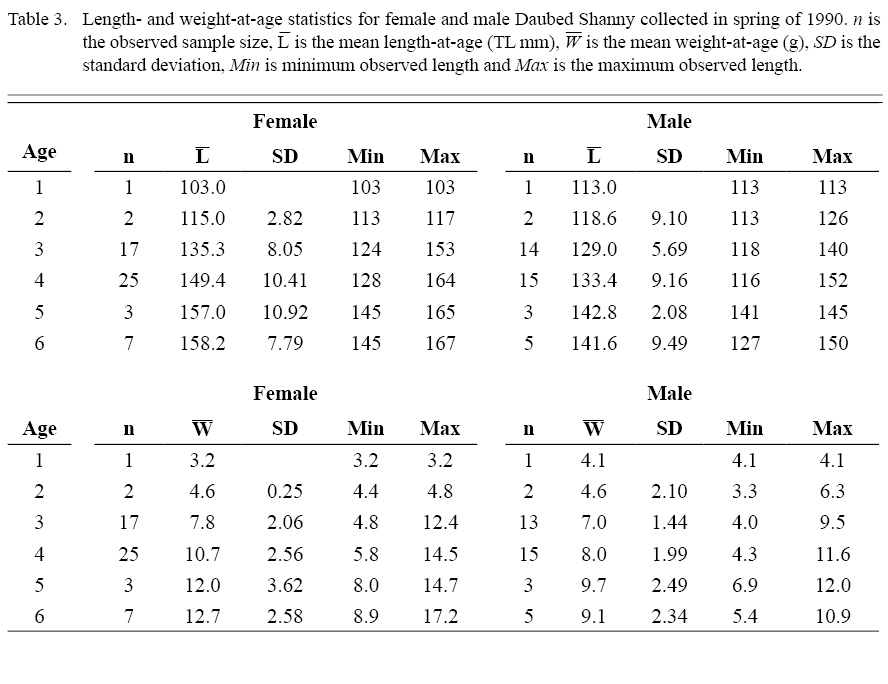
Table 3
The best information for determining trends in relative abundance of daubed shanny may come from the MEDMR and MADMF spring surveys. Those surveys had higher encounter rates (Supplemental Document Table SD and SE), no gear changes over time, vessel changes occurred once at the beginning of the time series examined, and survey trends were very similar and highly correlated. Based on those surveys, it appears that daubed shanny may be disappearing from the nearshore areas of Gulf of Maine given that relative abundance has been near zero or zero since 2016–2017 (no fish were caught in the MEDMR survey since 2021 and in the MADMF survey in 2023). The reasons why abundance has declined are unclear. One potential cause may be that rising water temperatures in the nearshore region (LaFreniere et al., 2023) and remaining Gulf of Maine (Townsend et al., 2023) are making areas less hospitable for a species adapted to living in cold waters. In fact, the rapid decline in daubed shanny abundance may be related to effects of a new baseline of warmer temperatures established in the Gulf of Maine in 2010 (Townsend et al., 2023). Being in the southern extent of its range, the species was likely already physiologically limited at historical temperatures (Sexton et al., 2009; Ern et al., 2023), and with the recent increases, its physiological thermal tolerances may have been pushed over upper thermal limits, affecting the species ability to survive. Declines in areal abundances of daubed shanny and other Arctic-boreal species are expected as warming waters push species northward (Fossheim et al., 2015).
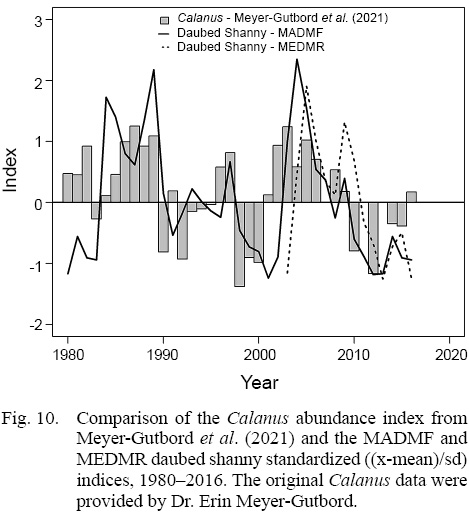
Fig. 10
An indirect effect of warming temperatures may also be causing the decline in daubed shanny. Post-larvae feed primarily on Calanus copepods and store lipids from this high-energy prey in a specialized lipid sac for winter survival (Meyer Ottesen et al., 2011). In the Gulf of Maine, Calanus finmarchicus was historically a dominant copepod (Pershing et al., 2005). After the establishment of the new thermal regime in 2010, C. finmarchicus abundance declined (Meyer-Gutbrod et al., 2021). Survival of daubed shanny post-larvae may have been greatly reduced because lower availability of Calanus translates to fewer encounters with this species, and lower encounters can affect a fish’s ability to capture enough prey to build energy reserves for overwintering (e.g., Letcher et al., 1996; Geissinger et al., 2021). Comparison of the MADMF and MEDMR survey indices with Calanus abundance index from Meyer-Gutbrod et al. (2021) (Fig. 10), suggests that a plausible link between Calanus and daubed shanny abundances exists.
Another indirect effect of warming temperatures is the potential for increased predation as distributions of marine predators change in response to warming waters. The biomasses of three hake species (Gulf of Maine red hake, Urophycis chuss, white hake, Urophycis tenuis, and northern silver hake, Merluccius bilinearis), identified as predators of daubed shanny through NEFSC stomach content analyses (Supplemental Document Table SI), have increased over time in the Gulf of Maine (Supplemental Document Fig. SA), likely related to distributional shifts in response to changing climate (Ney et al., 2011; LaFreniere et al., 2023). The decline of daubed shanny coincided with increases in hake biomass starting around 2006–2007 (Supplemental Document Fig. SA), suggesting potential interactions between daubed shanny and the predators. However, the very low occurrences of daubed shanny in stomachs of those and other fish predators (<0.15%; Supplemental Document Table SI) indicates that interactions may not be strong, and the observed inverse trends could be purely coincidental. Interestingly, warming waters and predation by longfin squid (Doryteuthis pealeii) appear responsible for the population collapse of the Gulf of Maine northern shrimp (Pandulus borealis), another boreal species with the southernmost extent of its range being the Gulf of Maine (Richards and Hunter, 2021). As suggested by one reviewer, there could be a potential predatory impact of marine mammals like the gray (Halichoerus grypus atlantica) and harbour (Phoca vitulina vitulina) seals that have also increased in abundance throughout the Gulf of Maine (Wood et al., 2019; Hayes et al., MS 2023), but a review of food habits showed that daubed shanny has not been found in the diet of those species in US waters (Waring et al., 2010; Lyssikatos and Wenzel, 2024; McCosker et al., 2024), suggesting a relationship may not exist.
Finally, commercial fishing in the Gulf of Maine could have a direct impact on daubed shanny abundance. The Gulf of Maine is an important fishing area for groundfishes such as Atlantic cod (Gadus morhua) and Atlantic haddock (Melanogrammus aeglefinus), and many gear types from bottom trawls to gillnets are used to target those species (Pol and Carr, 2000). Bycatch of non-targeted fish species in fisheries has been an issue for decades (Beutel et al., 2008) and it is possible to impact fish populations through bycatch mortality (Lawson et al., 2017). To determine if bycatch mortality could be an impactful cause, the NEFSC observer and at-sea monitoring database (https://www.fisheries.noaa.gov/inport/item/24111) was queried for any records of “shanny” collected between 2000 and 2024 from all areas, gear types and target fisheries. Only two records of catch (0.045 kg each) out of over 600 000 records were found, one for an unknown shanny and another for radiated shanny (Ulvaria subbifurcata), suggesting that bycatch impacts were unlikely a significant cause of the population decline of daubed shanny.
Depth, Temperature and Size Distributions
Andriyashev (1954) reported that daubed shanny were most abundant at depths from 50 to 240 m with temperatures between -1.6 and 2.0°C in the Barents Sea and as deep as 400 m off Greenland. In this study, the species was found in Gulf of Maine waters between 7 and 282 m within a wide range of much higher temperatures (1.3–15.7°C). Tyler (1971) also captured daubed shanny within a similar temperature range (1.2–10.7°C) in a Maine estuary. The average temperatures experienced in spring (all surveys: 4.0–4.4°C) and fall (NEFSC offshore and MADMF: 6.1–7.5°C) were close to the range of summer bottom temperatures (4–7°C) for daubed shanny captured in the White Sea (Andriyashev, 1954).
A seasonal change in depth distribution was noted as fish were caught mostly in shallow waters between 30 and 130 m in spring and in deeper waters ≥82 m in fall. In addition, daubed shanny were caught in the near-lowest temperatures available within most survey areas regardless of depth and season, suggesting the species has a thermal preference. The seasonal change in depth distribution could be the result of daubed shanny seeking specific temperatures, as has been observed in many fish species (Murawski, 1993; Henderson et al., 2017; Brazo et al., 2021), but this is unlikely because there was no concomitant shift of individuals of the same sizes found in spring to deeper waters in fall. However, the change in depth distribution was related to the appearance of small individuals (mean: 8 cm TL cm) not typically captured in spring. Daubed shanny post-larvae settle to the benthic habitat at sizes 70–100 mm CL (~76–108 mm TL) and mostly in waters >100 m (Meyer Ottesen et al., 2011; Murzina et al., 2012; Pekkoeva et al., 2018). Based on the observed size frequencies and depths-at-capture, the individuals captured in fall were likely benthic-settling post-larvae recruiting to offshore waters of the Gulf of Maine.
Fall catches and encounters of daubed shanny were greatly reduced in the nearshore (NEFSC inshore, MADMF, and MEDMR) surveys compared to spring. The catch of fish is dependent on gear selectivity (related to gear characteristics such as mesh size, net type, door design, etc.) and fish availability (related to the spatial distribution of fish relative to the distribution of the survey) (Maunder et al., 2014; McElroy et al., 2021). A change in gear selectivity was unlikely a cause of the seasonal change in daubed shanny abundance because gears used were relatively consistent across seasons in most surveys. A change in fish availability may be a more plausible cause of the seasonal differences in abundance; however, because little is known about daubed shanny habitat use and behavior, it is difficult to surmise likely hypotheses. Nevertheless, the disappearance of large (>11 cm TL) individuals in fall and their reappearance in spring, particularly in the offshore region of the NEFSC survey (see Supplemental Document Tables SJ-SM), suggests daubed shanny may be avoiding the gear by possibly moving to untowable habitats like rocky bottoms in fall. The fact that fish captured in the MADMF survey in fall had a similar size range as fish captured in spring and were still present, albeit in lower abundance, may be evidence to support that hypothesis.
Biology
In this study, we captured similar-sized individuals as the Meyer Ottesen et al. (2014) study but found that female daubed shanny comprised a higher percentage (weighted) of catches than males (58% versus 42%). The disparity in sex composition between the studies is likely due to differences in how fish were collected. In the Meyer Ottesen study, individual fish were collected over three years during April–October and were only sampled in deep water (150–400 m). As suggested by the authors, they may have missed females that could have been more abundant in shallower waters. Our sampling occurred within depths 48–60 m in Massachusetts waters where daubed shanny were concentrated in spring (May). Biases could have been produced by our limited sampling because fish were collected from only three tows, and we did not sample outside of Massachusetts waters. However, the consistency of higher female proportions across tows (0.54–0.6) suggests the pattern was real. Regardless, our results are closer in agreement with patterns of female dominance in other Stichaeidae species (Antonenko et al., 2004; Kolpakov and Klimkin, 2004; Kalchugin et al., 2006).
Meyer Ottesen et al. (2014) reported that daubed shanny males caught in Svalbard waters were larger and heavier than females, which agreed with the general pattern of larger and heavier males in other Stichaeidae species (Kalpakov and Klimkin, 2004; Kalchugin et al., 2006). In this study, we found the opposite for Gulf of Maine daubed shanny - females were significantly larger and heavier than males. We believe the difference is real and not created by our limited sampling. If the scope of spatial sampling in this study created bias, catches in surveys outside Massachusetts waters would be comprised of larger individuals, presumably males, than were captured in the MADMF survey. However, this was not the case. The MADMF survey tended to catch larger individuals compared to the other surveys (Figs. 6 and 7), and there was no evidence of higher fractions of larger individuals being caught or a trend of increasing size with increasing depth in the NEFSC inshore, MEDMR, or NEFSC offshore surveys (Supplemental Documents Table SN). Additionally, if bias was introduced by limited sampling of tows (3 out of 8 tows) and small sample size (101 out of 297 individuals) in this study, the estimated size composition from sampling might differ from the size composition of the entire survey, or sex-specific size compositions may vary among tows. Actually, there was little difference in estimated size compositions from the sampled tows and the entire survey (see Supplemental Document Fig. SB), and the pattern of smaller male size was consistent across tows (see Supplemental Document Fig. SC). Therefore, the reverse dimorphic patterns observed for Gulf of Maine daubed shanny were unlikely created by bias in our sampling.
Meyer Ottesen et al. (2014) found no difference in the gutted-weight versus caudal-length equation estimates between male and female daubed shanny from Svalbard waters. In this study, total weight versus total length equations for males and females were significantly different from each other, possibly due, in part, to the smaller sample size for males. The different measures of weight and length between the two studies make comparing and interpreting differences in equation estimates difficult. However, the equation slopes for female daubed shanny and for individuals pooled in the Gulf of Maine (3.34 for both) were close in magnitude to the slopes (3.43–3.46) estimated for both sexes and pooled individuals of the Svalbard population, which indicates both populations exhibit positive allometric growth and become disproportionately heavier with increasing length.
This study is the first to report age and growth estimates for daubed shanny in the Gulf of Maine. We aged both females and males to a maximum of 6 years, and females appear to grow faster and become larger than males. In comparison, Meyer Ottesen et al (2014) found females and males in Svalbard waters live a maximum of 10 and 12 years, respectively, and males tend to grow faster (after maturation) and larger than females. In addition, based on size frequencies and mean length-at-age comparisons, Gulf of Maine daubed shanny appear to grow faster and attain the same sizes earlier than fish from Svalbard waters.
The shorter life span and inter- and intra-population differences in body sizes and growth rates of females and males indicate that fish from the Gulf of Maine exhibit different life history characteristics than fish from the Svalbard population. These differences are likely adaptations of the species to living in much higher temperatures (1.3–15.7°C) at the southern extent of its range compared to the northern populations (-1.6–2.0°C; Andriyashev, 1954; Byrkjedal and Høines, 2007). Shorter lifespans and faster growth rates in lower latitude populations is a pattern observed in fishes with wide latitudinal distributions often attributed to increasing temperature and duration of the growing season as latitude decreases (Curtis and Shima, 2005; Heibo et al., 2005; Trip et al., 2014; Estlander et al., 2016; Riesch et al., 2018; Andrade et al., 2023). A within-species pattern of larger body sizes and faster growth rates of females in low latitude populations with a reversal to smaller sizes and slower growth rates in higher latitude populations has also been documented in several fish species, possibly the result of temperature influencing the relative costs of reproduction (Curtis and Shima, 2005; Estlander et al., 2016). Although not measured, daubed shanny in the Gulf of Maine likely experience higher natural mortality rates and mature earlier than northern populations because these life history characteristics covary with body size and growth rates, and increase and decrease, respectively, as latitude declines (Trip et al., 2014; Alvarez-Noriega et al., 2023). Our results demonstrate the importance of evaluating the life history of a species across its whole distributional range to understand the complex factors that may influence its survival and the species’ ability to respond to natural- and anthropogenic-induced environmental changes.
The prognosis for the continued presence of daubed shanny in the Gulf of Maine is not promising. The species is no longer captured in nearshore areas where it was historically abundant, and its disappearance may be linked to direct and indirect factors associated with increasing water temperatures. As temperatures continue to rise in the Gulf of Maine, as is projected (Brickman et al., 2021), the region will continue to become a very inhospitable environment for Arctic-boreal species like the daubed shanny. Our results give support to the conclusions of other studies (e.g., Richards and Hunter, 2021; LaFreniere et al., 2023) that major changes are occurring in the Gulf of Maine ecosystem due to climate change.
Acknowledgements
We thank Rebecca Peters of the Maine Department of Marine Resources, Paul Kostovick of the National Marine Fisheries Service, and Steve Wilcox of the Massachusetts Division of Marine Fisheries for providing access to the bottom trawl survey databases. Thanks to Brad Schondelmeier of the Massachusetts Division of Marine Fisheries for querying the NEFSC observer and at-sea monitoring database for shanny records, and to Katie Drew of the Atlantic States Marine Fisheries Commission for pointing out the similarities between daubed shanny and Calanus finmarchicus population fluctuations. Two anonymous reviewers provided helpful comments. The Maine Department of Marine Resources survey data were collected under award number NA22NMF4540361 from the National Oceanic and Atmospheric Administration, U.S. Department of Commerce. The statements, findings, conclusions, and recommendations are those of the authors and do not necessarily reflect the views of the Massachusetts Division of Marine Fisheries, the National Oceanic and Atmospheric Administration or the Department of Commerce.
References
Alvarez-Noriega, M., White, C. R., Kozlowski, J., Day, T., and Marshall, D. J. 2023. Life history optimization drives latitudinal gradients and response to global changes in marine fishes. PLOS Biology, 21(5): e3002114. https://doi.org/10.1371/journal.pbio.3002114.
Andrade, H., Vihtakari, M. and Santos, J. 2023. Geographic variation in the life history of lane snapper Lutjanus synagris, with new insights from the warm edge of its distribution. Journal of Fish Biology, 103: 950–964. https://doi.org/10.1111/jfb.15488
Andriyashev, A. P. 1954. Fishes of the northern seas of the U.S.S.R. Keys to the fauna of the U.S.S.R. Zoological Institute of the U.S.S.R Academy of Sciences, 53. Translated from Russian by the Israel Program for Scientific Translations, Jerusalem 1964, 266–269.
Antonenko, D. V., Pushchina, O. I., and Kalchugin, P. V. 2004. Distribution and biological features of the Long-snouted blenny Lumpella longirostris (Stichaeidae) in water of Primorye (the Sea of Japan). Journal of Ichthyology, 44: 757–751.
Azarovitz, T. R. 1981. A brief historical review of the Woods Hole Laboratory trawl survey time series. In: W. G. Doubleday and D. Rivard (Eds.), Bottom trawl surveys. Canadian Special Publications of Fisheries and Aquatic Sciences, 58: 62–67.
Beutel, D., Skrobe, L., Castro, K., Ruhle, P. Sr., Ruhle, P. Jr., O’Grady, J., and Knight, J. 2008. Bycatch reduction in the Northeast USA directed haddock bottom trawl fishery. Fisheries Research, 94: 190–198. https://doi.org/10.1016/j.fishres.2008.08.008
Bowman, R. E., Stillwell, C. E., W. L. Michaels, and M. D. Grosslein. 2000. Food of Northwest Atlantic fishes and two common species of squid. NOAA Tech. Memo. NMFS-F/, NE-155, 138 pp. https://doi.org/10.5962/bhl.title.4024
Brazo, A., Marques, R., Zimmermann, M., Aspillaga, E., Hereu, B., Saragoni, G., Mercière, A., Crec’Hriou, R., Mercader, M., Verdoit-Jarraya, M., Cadène F., and Lenfant, P. 2021. Seasonal influence on the bathymetric distribution of an endangered fish within a marine protected area. Scientific Reports, 11: 13342. https://doi.org/10.1038/s41598-021-92633-x
Brickman, D., Alexander, M. A., Pershing, A., Scott, J . D., and Wang, Z. 2021. Projections of physical conditions in the Gulf of Maine in 2050. Elementa Science of the Anthropocene, 9: 1–15. https://doi.org/10.1525/elementa.2020.20.00055
Bryant, R., Jones, I. L., and Hipfner, J. M. 1999. Responses to changes in prey availability by common murres and thick-billed murres at the Gannet Islands, Labrador. Canadian Journal of Zoology, 77: 1278–1287. https://doi.org/10.1139/z99-077
Byrkjedal, I., and Høines, A. 2007. Distribution of demersal fish in the south-western Barents Sea. Polar Research, 26: 135–151. https://doi.org/10.1111/j.1751-8369.2007.00030.x
Collette, B. B. 2002. Pricklebacks. Family Stichaeidae. In: B.B. Collette and G. Klein-MacPhee (Eds.), Bigelow and Schroeder’s Fishes of the Gulf of Maine, 476–477. Smithsonian Institution Press, Washington, D. C.
Curtis, T. D. and Shima, J. S. 2005. Geographic and sex-specific variation in growth of yellow-eyed mullet, Aldrichetta forsteri, from estuaries around New Zealand. New Zealand Journal of Marine and Freshwater Research, 39: 1277–1285. https://doi.org/10.1080/00288330.2005.9517392
Elliot, K. H., Woo, K., Gaston, A. J., Benvenuti, S., Dall’Antonia, L., and Davoren, G. K. 2008. Seabird foraging behavior indicates prey type. Marine Ecology Progress Series, 354: 289–303. https://doi.org/10.3354/meps07221
Elzey, S. P., Trull, K. J., and Rogers, K. A. MS 2015. Massachusetts Division of Marine Fisheries Age and Growth Laboratory: Fish Aging Protocols. Massachusetts Division of Marine Fisheries Technical Report TR-58, 43 p. https://www.mass.gov/files/documents/2016/08/oo/tr-58-full.pdf
Ern, R., Andreassen, A. H., and Jutfelt, F. 2023. Physiological mechanisms of acute upper thermal tolerance in fish. Physiology, 39: 141–158.
Estlander, S., Kahilainen, K. K., Horppila, J., Olin, M., Rask, M., Kubecka, J., Peterka, J., Riha, M., Huuskonen, H. and Murminen, L. 2016. Latitudinal variation in sexual dimorphism in life-history traits of a freshwater fish. Ecology and Evolution, 7: 665–673. https://doi.org/10.1002/ece3.2658.
Fogarty, M. J. 1985. Statistical considerations in the design of trawl surveys, FAO Fisheries Circular, No 786, 21 p.
Fossheim, M., Rimicerio, R., Johannesen, E., Ingvaldsen, R. B., Aschan, M. M., and Dolgov, A. V. 2015. Recent warming leads to a rapid borealization of fish communities in the Arctic. Nature Climate Change, 5: 673–678. https://doi.org/10.1038/nclimate2647
Geissinger, E. A., Gregory, R. S., Laurel, B. J., and Snelgrove, P. V. R. 2021. Food and initial size influence overwinter survival and condition of a juvenile marine fish (age-0 Atlantic). Canadian Journal of Fisheries and Aquatic Sciences, 78: 472–482. https://doi.org/10.1139/cjfas-2020-0142
Heibo, E., Maghagen, C., and Vollestad, L. A. 2005. Latitudinal variation in life-history traits in Eurasian perch. Ecology, 86: 3377–3386. https://doi.org/10.1890/04-1620
Henderson, M. E., Mills, K. E., Thomas, A. C., Pershing, A. J., and Nye, J. A. 2017. Effects of spring onset and summer duration on fish species distribution and biomass along the Northeast United States continental shelf. Reviews in Fish Biology and Fisheries, 27: 411–424. https://doi.org/10.1007/s11160-017-9487-9.
Hayes, S. A., Josephson, E., Maze-Foley, K., Rosel, P. E., McCordic, J., and Wallace, J. MS 2023. U. S. Atlantic and Gulf of Mexico marine mammal stock assessments 2022. NOAA Technical Memorandum NMFS-NE-304, 257 p.
Hovde, S. C., Albert, O. T., and Nilssen, E. M. 2002, Spatial, seasonal and ontogenetic variation in the diet of Northeast Arctic Greenland Halibut (Reinhardtius hippoglossoides). ICES Journal of Marine Science, 59: 421–437. https://doi.org/10.1006/jmsc.2002.1171
Howe, A. B., Correia, S. J., Currier, T. P., King, J., and Johnston, R. MS 2002. Spatial distribution of ages 0 and 1 Atlantic cod (Gadus morhua) off the eastern Massachusetts coast, 1978–1999, relative to habitat area of special concern. Massachusetts Division of Marine Fisheries Technical Report Series, TR-12. 35 p.
Jones, I. L., Rowe, S., Carr, S. M., Fraser, G., and Taylor, P. 2002. Different patterns of parental effort during chick-rearing by female and male thick-billed murres (Uria lomvia) at a low-Arctic colony. The Auk, 119: 1064–1074. https://doi.org/10.1093/auk/119.4.1064
Kalchugin, P. V., Pushchina, O. I., Panchenko, V. V., and Solomatov, S. F. 2006. The distribution and some biological features of Stichaeus grigorjewi (Stichaeidae) in the waters of Northern Primorye (the Sea of Japan). Journal of Ichthyology, 46: 447–453. https://doi.org/10.1134/S0032945206060051
Kolpakov, N. V., and Kilmkin, A. F. 2004. Specific features of biology of shannies Stichaeus grigorjewi and S. nozawae (Stichaeidae) in waters of Northern Primorye. Journal of Ichthyology, 44: 592–599.
Labansen, A. L., Lydersen, C., Haug, T., and Kovacs, K. M. 2007. Spring diet of ringed seals (Phoca hispida) from northwestern Spitsbergen, Norway. ICES Journal of Marine Science, 64: 1246–1256. https://doi.org/10.1093/icesjms/fsm090
LaFreniere, B. R., Peters, R., Donahue, B., McBride, R., and Moha, J. A. 2023. What the hakes? Correlating environmental factors with hake abundance in the Gulf of Maine. Journal of Northwest Atlantic Fisheries Science, 54: 17–29. https://doi.org/10.2960/J.v54.m742
Lawson, J. M., Foster, S. J., and Vincent, A. C. 2017. Low bycatch rates add up to big numbers for a genus of small fishes. Fisheries, 42: 19–33. https://doi.org/10.1080/03632415.2017.1259944
Leggett, W. C., and Carscadden, J. E. 1978. Latitudinal variation in reproductive characteristics of American Shad (Alosa sapidissima): evidence for population specific life history strategies in fish. Journal of Fisheries Research Board of Canada, 35: 1469–1478.
Lesage, V., Lair, S., Turgeon, S. and Béland, P. 2020. Diet of St. Lawrence estuary beluga (Delphinapterus leucas) in a changing ecosystem. The Canadian Field-Naturalist, 134(1): 21–35. https://doi.org/10.22621/cfn.v134i1.2421
Letcher, B. H., Rice, J. A., Crowder, L. B., and Rose, K. A. 1996. Variability in survival of larval fish: disentangling components with a generalized individual-based model. Canadian Journal of Fisheries and Aquatic Sciences, 53: 787–801. https://doi.org/10.1139/f95-241
Lyssikatos, M. C. and Wenzel, F. W. 2024. What bycatch tells us about the diet of harbour and gray seals and overlap with commercial fishermen. Frontiers in Conservation Science, 5: 1377673. https://doi.org/10.3389/fcosc.2024.1377673
Lumley, T. 2023. Package survey: analysis of complex survey samples. R package version 4.2. https://cran.r-project.org/web/packages/survey/survey.pdf
Lumley, T., and Scott, A. J. 2013. Two-sample rank tests under complex sampling. Biometrika, 100: 831–842. https://doi.org/10.1093/biomet/ast027
Lumley, T., and Scott, A. 2014. Tests for regression models fitted to survey data. Australian and New Zealand Journal of Statistics, 56(1), 1–14. https://doi.org/10.1111/anzs.12065
Maunder, M. N., Crone, P . R., Valero, J. L., and Semmens, B. X. 2014. Selectivity: theory, estimation, and application in fishery stock assessment models. Fishery Research, 158: 1–4. https://doi.org/10.1016/j.fishres.2014.03.017
McCosker, C. M., Olson, Z. H., and Ono, K. A. 2024. A comparative methodological approach to studying the diet of recovering marine predator, the grey seal (Halichoerus grypus). Canadian Journal of Zoology, 102: 182–194. https://doi.org/10.1139/cjz-2023-0104
McElroy, W. D., Blaylock, J., Shepherd, G. R., Legault, C. M., Nitschke, P. C.,and Sosabee, K. A. 2021. Comparison of a bottom longline survey and a bottom trawl survey for 2 groundfish species in the Gulf of Maine to evaluate habitat-related availability of large fish. Fishery Bulletin, 119: 231–242.
https://doi.org/10.7755/FB.119.4.3
Mecklenburg, C. W., Møller, P. R., and Steinke, D. 2011. Biodiversity of arctic marine fishes: taxonomy and zoogeography. Marine Biodiversity, 41: 109–140. https://doi.org/10.1007/s12526-010-0070-z
Meyer-Gutbrod, E. L., Greene, C. H., Davies, K. T. A., and Johns, D. G. 2021. Ocean regime shift is driving collapse of the North Atlantic right whale population. Oceanography, 34: 23–31. https://doi.org/10.5670/oceanog.2021.308
Meyer Ottesen, C. A., Hop, H., Christiansen, J. S., and Falk-Petersen, S. 2011. Early life history of the daubed shanny (Teleostei: Leptoclinus maculatus). Marine Biodiversity, 41: 383–394. https://doi.org/10.1007/s12526-010-0079-3
2014. Growth of daubed shanny (Teleostei: Leptoclinus maculatus) in Svalbard waters. Polar Biology, 37: 809–815. https://doi.org/10.1007/s00300-014-1481-2
2018. Reproduction and sexual dimorphism of Daubed Shanny (Teleostei: Leptoclinus maculatus) in Svalbard waters. Polar Biology, 41: 1867–1880. https://doi.org/10.1007/s00300-018-2328-z
Miller, T. J., Das, C., Politis, P. J., Miller, A. S., Lucey, S. M., Legault, C. M., Brown, R.W., and Rago, P. J. MS 2010. Estimation of Albatross IV to Henry B. Bigelow calibration factors. NEFSC Ref. Doc. 10–05.
Miller, T. J. 2013. A comparison of hierarchical models for relative catch efficiency based on paired-gear data for US Northwest Atlantic fish stocks. Canadian Journal of Fisheries and Aquatic Sciences, 70: 1306–1316. https://doi.org/10.1139/cjfas-2013-0136
Murawski, S. A.1993. Climate change and marine fish distributions: forecasting from historical analogy. Transactions of the American Fisheries Society, 122: 647–658. https://doi.org/10.1577/1548-8659(1993)122<0647:CCAMFD>2.3.CO;2
Murzina, S. A., Meyer Ottesen, C. A., Falk-Petersen, S., Hop, H., Nemova, N. N., and Poluektova, O. G. 2012. Oogenesis and lipids in gonad and liver of daubed shanny (Leptoclinus maculatus) females from Svalbard waters. Fish Physiology and Biochemistry, 38: 1393–1407. https://doi.org/10.1007/s10695-012-9627-z
Nelson, G. A. 2014. Cluster sampling: a pervasive, yet little recognized survey design in fisheries research. Transactions of the American Fisheries Society, 143: 926–938. https://doi.org/10.1080/00028487.2014.901252
Ney, J. A., Joyce, T . M., Kwon, Y., and Link, J. S. 2011. Silver hake tracks changes in Northwest Atlantic circulation. Nature Communications, 2: 412–417. https://www.doi.org/10.1038/ncomms1420.
Northeast Fisheries Science Center. MS 1997. Report of the 24th Northeast Regional Stock Assessment Workshop (24th SAW): stock Assessment Review Committee (SARC) consensus summary of assessments. Northeast Fisheries Science Center Reference Document, 97–12, 291 p.
Pekkoeva, S. N., Murzina, S. A., Ieshko, E. P., Nefedova, Z. A., Falk-Petersen, S., Berge, J., Lønne, O., and Nemova, N. N. 2018. Ecological groups of the daubed shanny Leptoclinus maculatus (Fries,1938), an Artco-boreal species, regarding growth and early development. Russian Journal of Ecology, 49: 253–259. https://doi.org/10.1134/S1067413618030074
Pekkoeva, S. N., Kondakova, E. A., Falk-Petersen, S., Berge, J., and Murzina, S. A. 2023. Ontogenetic changes in the body structure of the Arctic fish Leptoclinus maculatus. Scientific Reports, 13: 3688. https://doi.org/10.1038/s41598-023-30251-5
Perry, R. I., and Smith, S. J. 1994. Identifying habitat associations of marine fishes using survey data: an application to the northwest Atlantic. Canadian Journal of Fisheries and Aquatic Sciences, 51: 589–602. https://doi.org/10.1139/f94-061
Pershing, A. J., Greene, C. H., Jossi, J. W., O’Brien, L., Brodziak, J. K. T., and Bailey, B. A. 2005. Interdecadal variability in the Gulf of Maine zooplankton community with potential impacts on fish recruitment. ICES Journal of Marine Science, 62: 1511–1523. https://doi.org/10.1016/j.icesjms.2005.04.025
Pol, M., and Carr, H. A. 2000. Overview of gear developments and trends in the New England commercial fishing industry. Northeastern Naturalist, 7: 329–336. https://doi.org/10.1656/1092-6194(2000)007[0329:OOGDAT]2.0.CO;2
Richards, R. A., and Hunter, M. 2021. Northern shrimp Pandulus borealis population collapse linked to climate-driven shifts in predator distribution. PLos ONE, 16(7): e0253914. https://doi.org/10.1371/journal.pone.0253914
Riesch, R., Martin, R. A., Diamond, S. E., Jourdan, J., Plath, M., and Langerhans, R. B. 2018. Thermal regime drives a latitudinal gradient in morphology and life history in a livebearing fish. Biological Journal of the Linnean Society, 125: 126–141. https://doi.org/10.1093/biolinnean/bly095
Scott, W. B., and Scott, M. G. 1988. Atlantic Fishes of Canada. Canadian Bulletin of Fisheries and Aquatic Sciences, 219. 731 p.
Seigel, S. 1956. Nonparametric Statistics for Behavioral Sciences. McGraw-Hill, New York, NY. 312 p.
Sexton, J. P., McIntyre, P. J., Angert, A. L., and Rice, K. J. 2009. Evolution and ecology of species range limits. The Annual Review of Ecology, Evolution and Systematics, 40: 415–436. https//doi.org/10.1146/annurev.ecolsys.110308.120317
Shepherd, G., and Grimes, C. B. 1983. Geographic and historic variations in growth of weakfish, Cynoscion regalis, in the Middle Atlantic Bight. US Fishery Bulletin, 81: 803–813.
Sherman, S. A., Stepanek, K. and Sowles, J. MS 2005. Maine-New Hampshire inshore groundfish trawl survey: procedures and protocols. https://www.maine.gov/dmr/sites/maine.gov.dmr/files/docs/proceduresandprotocols.pdf
Sissenwine, M. P., and Bowman, E. W. 1978. An analysis of some factors affecting the catchability of fish by bottom trawls. International Commission for the Northwest Atlantic Fisheries Research Bulletin, 13: 81–87.
Sokal, R. R., and Rohlf, F. J. 1995. Biometry, 3rd edition. Freeman, New York.
Thompson, S. K. 2002. Sampling. John Wiley & Sons, Inc. New York. 367 p.
Townsend, D. W., Pettigrew, N. R., Thomas, M. A., and Moore, S. 2023. Warming waters of the Gulf of Maine: the role of shelf, slope, and Gulfstream water masses. Progress in Oceanography, 125: 103030. https://doi.org/10.1016/j.pocean.2023.103030
Trip, E. D. L., Clements, K. D., Raubenheimer, D., and Choat, J. H. 2014. Temperature-related variation in growth rate, size, maturation and life span in a marine herbivorous fish over a latitudinal gradient. Journal of Animal Ecology, 83: 866–875. https://doi.org/10.1111/1365-2656.12183
Tyler, A. V. 1971. Periodic and resident components in communities of Atlantic fishes. Journal of the Fisheries Research Board of Canada, 28: 1727–1732. https://doi.org/10.1139/f71-139
Waring, G. T., Gilbert, J. R., Belden, D., Van Atten, A., and DiGiovanni., G. 2010. A review of the status of harbour seals (Phoca vitulina) in the Northeast United States of America. NAMMCO Scientific Publications, 8: 191–212. https://doi.org/10.7557/3.2685
Wood, S. A., Murray, K. T., Josephson, and Gilbert, J. 2019. Rates of increase in gray seal (Halichoerus grypus atlantica) pupping at recolonized sites in the United States, 1988-2019. Journal of Mammology, 101: 121–128. https://doi.org/10.1093/jmammal/gyz184
Citation: Nelson, G.A., Duprey, K.L., and Elzey, S.P. 2024. Aspects of the Population Dynamics and Biology of the Daubed Shanny (
Leptoclinus maculatus) from the Gulf of Maine.
J. Northw. Atl. Fish. Sci.,
55: 11–29. https://doi.org/10.2960/J.v55.m747