J. Northw. Atl. Fish. Sci., Vol. 53: 57–77
Richard S. McBride*1![]()
 , George A. Maynard1, 2, Scott P. Elzey3, Daniel R. Hennen1, Emilee K. Tholke4, Jocelyn M. Runnebaum5, and Christopher H. McGuire5
, George A. Maynard1, 2, Scott P. Elzey3, Daniel R. Hennen1, Emilee K. Tholke4, Jocelyn M. Runnebaum5, and Christopher H. McGuire5
1National Marine Fisheries Service (NMFS), Northeast Fisheries Science Center (NEFSC),
166 Water Street, Woods Hole, MA (richard.mcbride@noaa.gov, george.maynard@noaa.gov,
daniel.hennen@noaa.gov, emilee.tholke@noaa.gov)
2Formerly with the Cape Cod Commercial Fishermen’s Alliance,
1566 Main Street, Chatham, MA 02633
3Massachusetts Division of Marine Fisheries,
30 Emerson Avenue, Gloucester, MA 01930
(scott.elzey@mass.gov)
4IBSS Corporation, under contract with NMFS, NEFSC1
5The Nature Conservancy, 4245 North Fairfax Drive Suite 100, Arlington, Virginia, 22203
(jocelyn.runnebaum@tnc.org, cmcguire@tnc.org)
*Corresponding Author.
McBride, R.S., Maynard, G.A., Elzey, S.P., Hennen, D.R., Tholke, E.K. Runnebaum, J.M. and McGuire, C.H. 2022. Evaluating growth dimorphism, maturation, and skip spawning of Atlantic halibut in the Gulf of Maine using a collaborative research approach. J. Northw. Atl. Fish. Sci., 53: 57–77. https://doi.org/10.2960/J.v53.m736
Abstract
The data-limited nature of Atlantic halibut (Hippoglossus hippoglossus) in U.S. waters hampers evaluation of what may be a slow but steady rebuilding pattern. Here, we collaborate with the commercial fishery to design and implement a multi-gear sampling program that collected 100s of biological samples from throughout the Gulf of Maine in a five-year period, 2014–2018. Examination of sectioned otoliths revealed a maximum age of 12 years (females) and 13 years (males); in comparison, Atlantic halibut as old as 40–50 years have been collected elsewhere in the western North Atlantic. Growth modeling confirmed sexual dimorphism, with a larger asymptotic length (L∞) for females (214 cm fork length [FL]) than males (195 cm FL). Estimates of median female length at maturity, L50, of 128 cm FL (124–132 cm, 95% confidence limits), and median female age at maturity, A50, of 9.6 years old (9.0–10.8 years), were longer and older than previous reports for the Gulf of Maine, likely resulting from our use of histological instead of macroscopic methods to classify maturity. Histology demonstrated that vitellogenesis initiated in individuals in spring, nearly a year prior to spawning, which allowed us to identify first-time (primiparous) spawners and provided the first potential evidence of skip spawning for this species. Finally, an index was developed to track the proportion of potentially mature females in the fishery, which showed an increasing trend; this qualitative tool may prove useful in a data-limited environment for evaluating the relative stock status of Atlantic halibut.
Keywords: age, cooperative research, life history, reproduction, Hippoglossus hippoglossus
PDF | Supplementary Materials 1 | Supplementary Materials 2
Download Citation Data

Citation to clipboard
 Reference management software (Endnote, Mendeley, RefWords, Zotero & most other reference management software)
Reference management software (Endnote, Mendeley, RefWords, Zotero & most other reference management software)
LaTex, BibDesk & other specific software
Introduction
The current stock status of Atlantic halibut, Hippoglossus hippoglossus, in U.S. waters is unknown but is considered well below target levels based on qualitative evaluation of the stock and historical evidence of much higher abundance (Blaylock and Legault, 2012; Hennen, 2020). Bottom trawls exhibit relatively poor catchability for Atlantic halibut, and persistently low catches in standardized fishery-independent bottom trawl surveys in U.S. waters confounds the detection of abundance trends (Hovgård and Riget, 1992; Zwanenburg et al., 2003; Rago, 2018; DFO, 2020). Evidence of rebuilding is limited but includes: 1) managed fishery catches that have steadily increased from about 40 metric tons (mt) annually during the fishing years 2002–2004 to about 140 mt during 2017–2019, and 2) a time series of commercial catch standardized to fishing effort that shows a stable or slightly increasing trend (Hansell et al., 2020). In contrast to the U.S. situation, Atlantic halibut in Canadian waters are certified by the Marine Stewardship Council. Much higher landings are reported, between 2500 and 4100 mt each year since 2010 (DFO, 2020), and the value of this fishery in Canada has increased seven-fold since the early 2000s (Shackell et al., 2021).
Atlantic halibut are managed and assessed independently by the United States and Canada with conflicting evidence these stocks may (Kersula and Seitz, 2019; Liu et al., 2019) or may not (Seitz et al., 2016; Shackell et al., 2016) be connected. Recent genomic evidence suggests two genotypic stocks in the western North Atlantic: one across the North American continental shelf region and another in the semi-enclosed Gulf of St. Lawrence (Kess et al., 2021). Such genetic structure is overlaid by additional spatial structure in life history traits and migratory contingents (Shackell et al., 2021).
U.S. Atlantic halibut is likely to remain data limited, for some time, regarding assessment and management. Life history traits typically provide useful context for such data-limited situations. For example, heavily overfished stocks are characterized by size and age truncation, with corresponding loss in reproductive potential (Hixon et al., 2014; Barnett et al., 2017). In the case of Atlantic halibut, females can live to 38 and males to 50 years (Armsworthy and Campana, 2010), but in U.S. waters, Sigourney et al., (2006) observed few fish older than 13 years.
Confounding a direct interpretation of age truncation as a result of fishing pressure, Atlantic halibut exhibit broad geographic variability in life history traits. Growth rates are faster and sizes at maturity are smaller in warmer, southern waters (Armsworthy and Campana, 2010; Shackell et al., 2019; Shackell et al., 2021). Atlantic halibut in the Gulf of Maine may experience faster growth rates because of higher temperatures than in most Canadian waters, and they appear to exhibit sexual dimorphism as evident in most flatfish species, but growth modeling of fish captured in the Gulf of Maine has not been reported in the literature (Sigourney et al., 2006; Shackell et al., 2019).
Some aspects of Atlantic halibut reproduction are not well understood in U.S. waters but general reproductive principles are known based on halibut from other areas. Atlantic halibut is an iteroparous, batch spawner with group-synchronous oocyte development with respect to vitellogenesis (Norberg et al., 1991; Neilson et al., 1993). The final stages of oocyte maturation appear to begin in January – indicating the initiation of winter spawning – becoming transparent and reaching sizes of 2.3–3.4 mm (Haug and Gulliksen, 1988). Haug and Gulliksen (1988) estimated annual fecundity of Atlantic halibut to range from 0.5 to 7.0 million eggs for females from 132 to 195 cm total length. Elevated temperatures have been demonstrated to delay spawning and reduce quantity and quality of eggs for Atlantic halibut (Brown et al., 2006).
In this study, we provide new life history parameters for Atlantic halibut at the southern range of its geographic distribution in the western North Atlantic. We overcame a chronic issue of low sample sizes by partnering with fishermen, and used a variety of fishing gears, which resulted in large numbers of fish of all sizes, both sexes, and year round throughout the Gulf of Maine (Read and Hartley, 2006; Fairclough et al., 2014; Thiel et al., 2014). We used best practices for ageing fish, including a direct comparison of thin-sectioned otoliths to whole otoliths (Karlson et al., 2013). We also applied gonad histology as a best practice to evaluate reproductive maturity and to reveal evidence of primiparity and skip spawning (Rideout and Tomkiewicz, 2011; McBride et al., 2013). Finally, to aid in future evaluation of the population, we propose the use of an index that approximates the proportion of mature females in the fishery, as a qualitative tool for evaluating the relative stock status.
Materials and methods
Collaborative sampling
A collaborative research approach between federal, state, and industry partners sampled Atlantic halibut throughout the Gulf of Maine. The largest single so urce of biological samples came from a novel, collaborative fishery-dependent sampling program lead by the Cape Cod Commercial Fishermen’s Alliance (CCCFA) and The Nature Conservancy (TNC), who used multiple bottom-tending gear types (trawl, longline, and gillnet), fishing predominantly east of Cape Cod (Fig. 1; sample sizes tabulated in Supplemental Materials). Fishermen were compensated for each complete halibut sample, under conditions set forth in an Exempted Fishing Permit (EFP) by NOAA fisheries, and issued to 23 collaborating fishing businesses. Researchers trained collaborating fishermen through in-person training sessions, written protocols, and with a ‘how-to’ video (CCCFA, 2018) recorded by participating fishermen. Fishermen were provided sampling kits including tools, datasheets, a spring scale, and various sampling containers. Northeast multispecies regulations limit fishermen to landing one legal sized (> 41 inches, 104 cm) halibut per trip; under the EFP, the number of samples per day was increased to five in order to maximize sample collection, although it was extremely rare to receive five samples from a single trip.
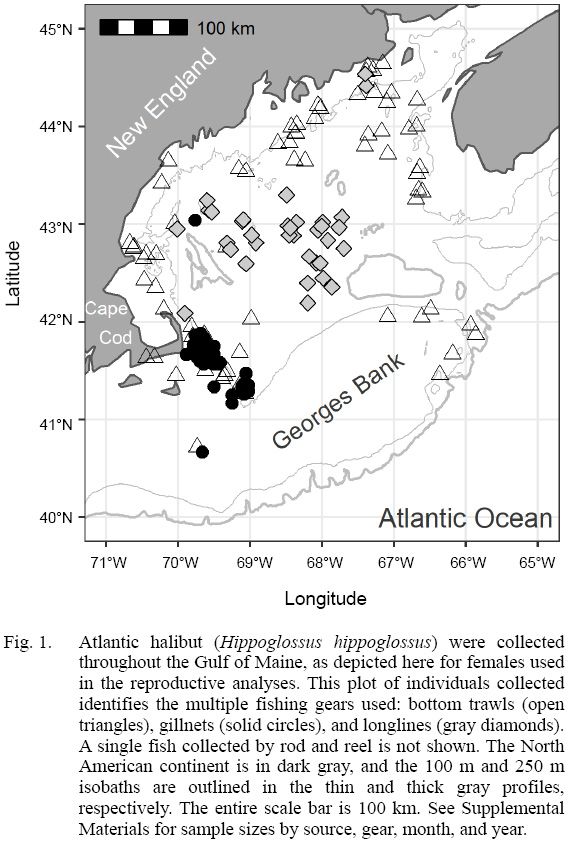
Fig. 1
Additional halibut were obtained from several other sources: Maine’s commercial fishery; the Maine and New Hampshire inshore bottom trawl survey; the Northeast Fisheries Science Center’s (NEFSC) Ecosystem Survey Branch bottom trawl survey (Politis et al. 2014); the NEFSC’s Cooperative Research Branch (CRB) bottom longline survey (McElroy et al. 2019); an industry-based bottom trawl survey in partnership with the Massachusetts Division of Marine Fisheries; and lastly, the NEFSC CRB Study Fleet Program.
Fish were collected as early as 2014–16, and sampling rates increased markedly in 2017–18 as a result of the partnership with CCCFA and TNC. Fish were collected in all months of the year except March during the 5-year period 2014–2018 (Supplemental Materials).
Fish were initially processed at sea and samples were stored on ice to be processed and preserved in the laboratory within 48 hours. Fork length (FL) was measured and reported to the nearest 1 cm. Total mass (TM) was measured to the nearest 1 g by fishery-independent sources or 500 g by fishery-dependent sources, and reported in kg. Ovary mass (OM) was recorded to the nearest g, and the gonadosomatic index was calculated as GSI = OM/(TM-OM) × 100. Sagittal otoliths were excised and stored dry in envelopes for ageing. Approximately 1 cm3 piece of tissue was excised from the middle of the ovary and fixed in 10% neutral-buffered formalin for gonad histology.
Otolith ageing
Age determinations were made on whole sagittal otoliths prior to sectioning for subsequent determinations. Halibut sagittal otoliths differ slightly in shape between the eyed side of the fish and the blind side. When possible, both otoliths from each fish were examined side by side. The asymmetrical (eyed side) otolith tends to be thinner and wider which yields clearer annuli during whole otolith ageing. Whole otoliths were immersed in mineral oil and the distal surface was examined with reflected light. Oil was wiped off after ageing to prevent clearing of the otoliths.
After each sample had been aged whole, the otoliths were embedded in epoxy and thin sectioned through the core with a low-speed diamond wafering saw. These sections were then affixed to glass microscope slides and examined either with reflected (Fig. 2) or transmitted light. The age readers preferred the use of reflected light, except in the oldest fish where increased magnification was needed. The symmetrical otolith was preferred for age determination of thin sections as they are thicker, yielding to better separation of annuli in the primary reading plane along the edge of the sulcal groove.
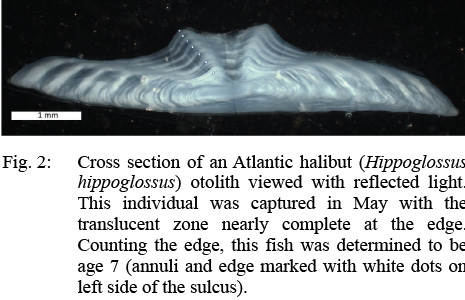
Fig. 2
All structures (n = 421) were assigned an age by two independent readers, with no knowledge of fish size, sex, or previously assigned ages. For structures in which there was disagreement in age between readers, a third joint reading was conducted to reach a consensus age. If a consensus could not be reached, the sample was discarded.
Although otolith ageing with a thin section method has been validated by Armsworthy and Campana (2010), we had not aged this species previously, so we compared ages from surface reads of whole otoliths to sectioned otoliths, and a subsample (n = 92) of the otoliths (whole and sectioned) was aged twice by each reader to assess within reader precision. Estimates of age precision included coefficient of variation (Chang, 1982), percent agreement, and percent agreement + 1 year. Precision estimates were generated between readers (n = 327) and within readers (n = 92) for a subset of the structures. Tests of symmetry (Bowker’s, Evans and Hoenig’s, McNemar’s, as described by McBride [2015]) were performed using the FSA package in R (Ogle et al., Version 0.8.30, 2020). Due to difficulty reading whole otoliths and very low reader confidence, consensus ages were only reached for the subset of whole otoliths.
Final age assignments were based on the number of annuli counted on the sectioned otoliths, the capture date, and the amount of otolith growth after the final completed annulus. This grouped all fish spawned in a given calendar year into the same age group. A birthdate of December 1st was used, based on the literature and evidence presented herein, to calculate biological ages for use in growth modeling.
The von Bertalanffy and Gompertz growth models were evaluated for sex-specific growth of Atlantic halibut. Models were varied to allow each combination of model parameters to be fixed or variable between sexes. Size and age data of 93 males and 297 females were fit to both models and evaluated using Akaike’s Information Criterion (AIC) within R software (version 3.6.3) (Supplemental materials).
Our ability to evaluate gear bias was limited by small sample sizes for long line and rod and reel. However, length at age for ages 4–8 were compared between gillnet-captured fish and bottom-trawl-captured fish using Student’s t tests.
Chapman-Robson mortality estimates were calculated using the FSA package in R (Ogle et al., Version 0.8.30, 2020). Mortality was calculated with all data combined, by sex, and by gear, including 95% confidence intervals.
Ovarian Histology and Oocyte Staging
Subsamples of fixed ovarian tissue were trimmed to < 4 mm thickness, placed in a cassette, and returned to formalin until histology processing. These subsamples were prepared using standard paraffin embedding techniques (McBride et al., 2013), including sectioning the tissue using a rotary microtome set to 5 µm, mounting on microscope slides, and staining with Schiffs-Mallory trichrome. Histology slides were viewed (20–400x) on a monitor using a microscope and digital camera.
Descriptions for oocyte stages, including post-ovulatory follicles (POFs) and oocyte atresia, were modified from Neilson et al. (1993) for Atlantic halibut; Kennedy et al. (2011) for Greenland halibut, Reinhardtius hippoglossoides; Press et al.(2014) for winter flounder, Pseudopleuronectes americanus; and Fish (2020) for Pacific halibut, Hippoglossus stenolepis (See also Supplemental Materials). At their most advanced, primary growth (PG) germ cells were small (< 200 µm) with nucleoli scattered along the periphery of the nucleus (Fig. 3). Development of early cortical alveoli (EC) in the germ cell were the first cytoplasmic inclusions noted, appearing initially as scattered, unstained vacuoles on the periphery of the cell. A late cortical alveoli (LC) germ cell stage was recognized once a biphasic dark-light ring of inclusions developed around the periphery of the cytoplasm. An early vitellogenesis (V1) stage emerged as orange inclusions appeared proximal to the outer ring of cortical alveoli. Vitellogenic inclusions spread until the cytoplasm was ‘fully yolked’ (V2). Histology plate images were scanned using the Grundium Ocus slide scanner and images were taken using Aperio ImageScope software (Figs. 3, 4).
Evidence of oocyte maturation — either migration or breakdown of the nucleus or hydration of the cell — was not observed. Evidence of ovulation was rare, with only the oldest stages of post-ovulatory follicles observed. Follicular atresia was identified as early (alpha) or late (beta), and ranked by extent, but offered little additional information to classify maturity. Tunica (gonad wall) thickness was measured to the nearest micron, as an index of maturity, when present in the histology section. Measurement of the tunica occurred at an intact section of the tissue, away from edges caused by excision of the tissue (see Fig. 4 and Supplemental Materials).
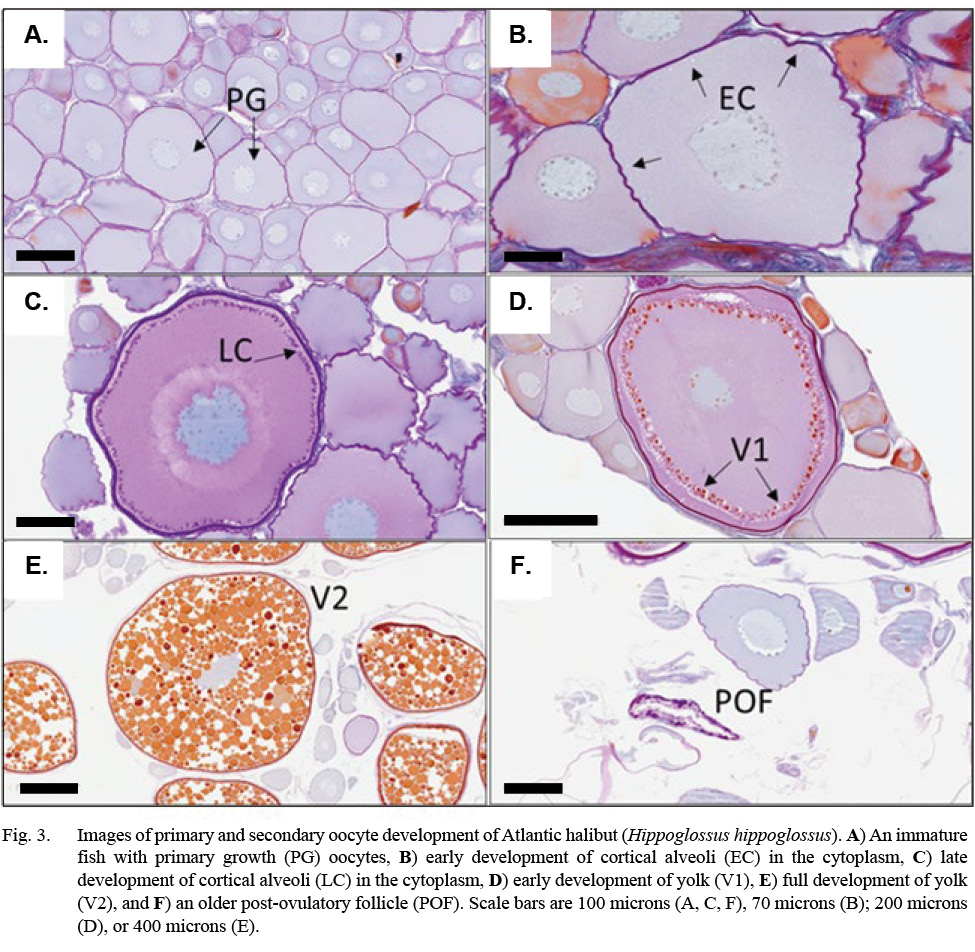
Fig. 3
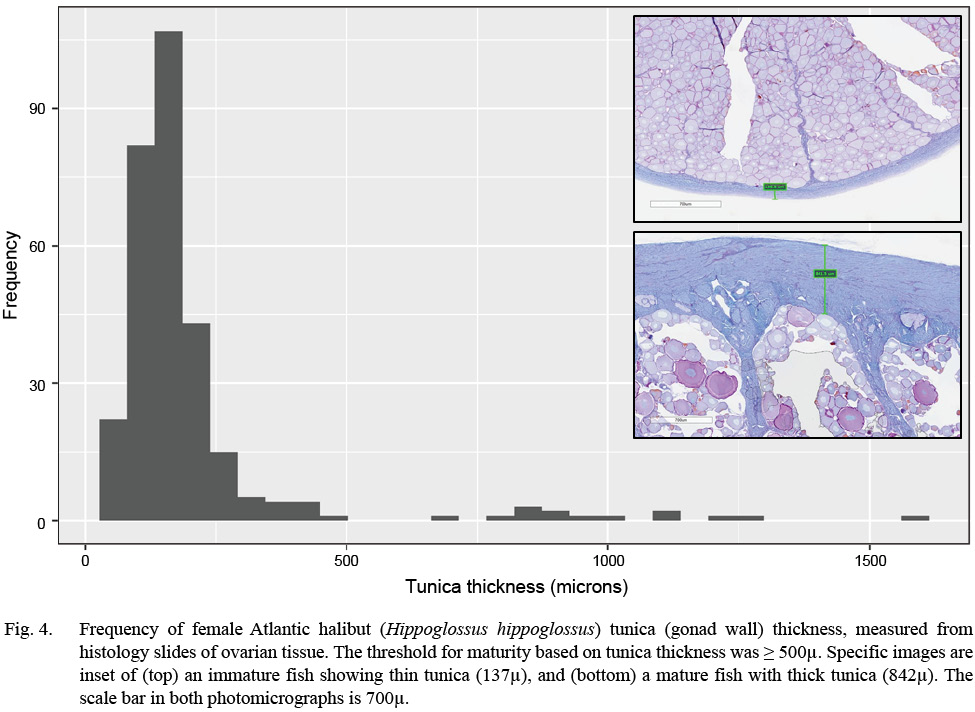
Fig. 4
Later, we refer to oocyte stage-specific maximum oocyte diameters. These were measured from images of approximately 300 oocytes per female suspended in water, as captured using a microscope and camera (Leica M26 scope and Leica DFC295 camera). Image analysis software (ImageJ with the plugin ObjectJ) was used to manually measure the oocyte diameters. The most advanced oocyte stage (MAOS) of these whole oocytes was identified from a paired sample of ovarian histology.
Maturity classification, ageing, and modelling
Maturity was classified following criteria initially developed for Greenland halibut (Kennedy et al., 2011) and winter flounder (Press et al., 2014). In the former, vitellogenesis required more than a year to complete, whereas in the latter, vitellogenesis started 10–12 months prior to spawning. Atlantic halibut were classified as immature if they did not have vitellogenic oocytes, any POFs, or a thin tunica (< 500 µ). They were classified as mature if they had vitellogenic oocytes (MAOS V1 or V2), POFs of any age, or a thick tunica. The use of a thick or thin tunica is directed at distinguishing all immature classes from resting fish, the latter of which is a mature class and often confused with immature fish macroscopically (e.g., McBride et al., 2013). Support of the specific threshold of 500 µm is presented elsewhere in this paper (Fig. 4).
The binomial, logistic model — logit (mature) = ea+bX/(1 + ea+bX) — was used to estimate maturity parameters, where a and b are estimated and X is length or age. Confidence limits (95%) were estimated by bootstrapping the data using the sizeMat package in R (Torrejon-Magallanes, 2020).
Changes in Proportion Mature Over Time
In the absence of an effective fishery-independent survey for Atlantic halibut in U.S. waters, fishery-dependent data have been used for stock assessment (Hennen, 2020). In particular, commercial discard rates have been used as an index of abundance. As discarded fish are typically small, a new index was developed to track the proportion of fish landed larger than the L50, determined herein as 128 cm, as measured by port samplers.
Atlantic halibut regulations in the United States have restricted fishermen to one landed fish per trip since 1999, and the minimum size of retention was adjusted from 36 to 41 inches (91.4, 104 cm) in 2009, which starts our time series. In practice, this results in some unknown degree of highgrading where the largest fish encountered is the most likely to be landed. Therefore, the size composition of landed fish may be reasonably indicative of trends in the average maximum size of fish encountered. The annual proportions were estimated as the proportion of fish larger than the L50 estimate, using U.S. catches since 2009, and the time series was fit with a generalized linear model (GLM). The data are binomially distributed, where fish > L50 were given a 1 and fish < L50 were given a 0. The model used year to predict the proportion mature.
Comparing the proportion mature in the Canadian Scotian shelf (4WXZ) stock of Atlantic halibut to the U.S. stock was of interest as the CA stock is relatively data rich and has increased over recent years (DFO, 2020). Fixed station longline survey data from area 4WXZ was also fit to a GLM using year to predict proportion > L50. Survey data in this case were preferred as there is reason to believe that the selectivity of fishers in area 4WXZ has changed, in order to better target larger Atlantic halibut (den Heyer pers. com).
Results
Collaborative, multigear sampling
Atlantic halibut were collected throughout the Gulf of Maine (Fig. 1). Bottom trawls collected Atlantic halibut in all years, 2014–2018, all around the Gulf of Maine, in both U.S. and Canadian waters, with some samples on the northeast peak of Georges Bank and offshore of southern New England (Supplemental Materials). Gillnets operated by the CCCFA focused their fishing offshore of Cape Cod, sampling in 2017–18. Longline sampling collected Atlantic halibut in all years, primarily in the deeper portions of the Gulf of Maine.
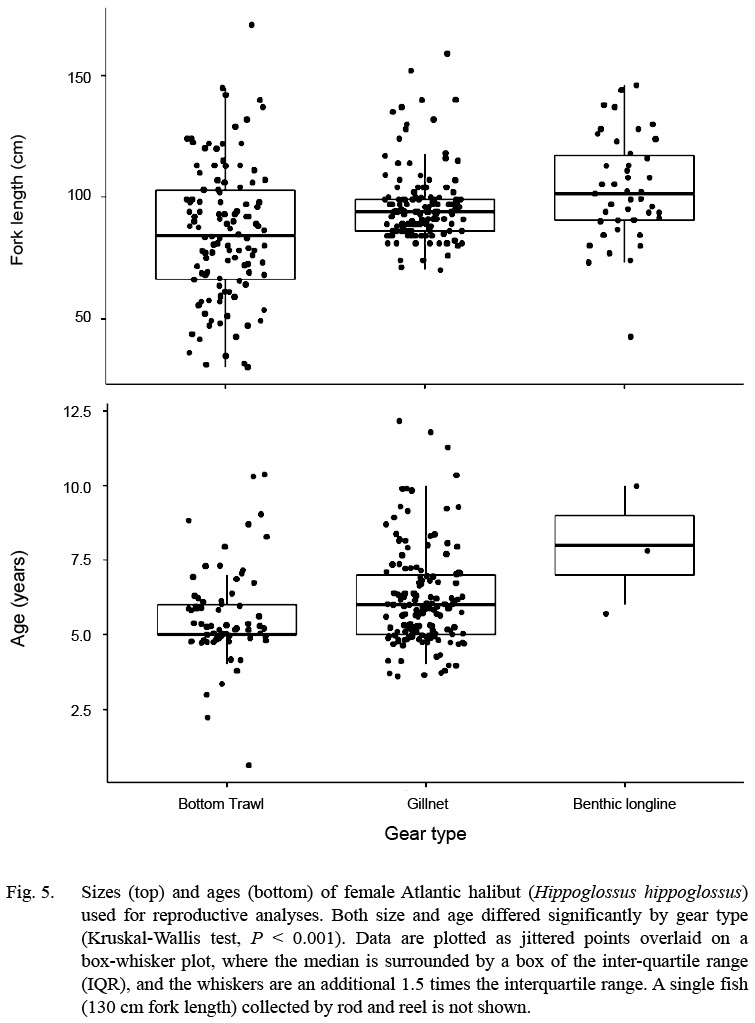
Fig. 5
A wide size range of fish was collected, and the size ranges differed between gears. Fish caught by bottom trawl had the smallest median size (84 cm fork length), but the widest range of sizes (30–171 cm; n = 118; Fig. 5). Fish caught by gillnet had the narrowest inter-quartile range, and the longline gear had the highest median. Although, longline gear is typically used to target larger Atlantic halibut in commercial fisheries of the Gulf of Maine and Scotian Shelf, the longlines used here were rigged for groundfish (cod, haddock, pollock) with small (12/0 size) hooks and squid bait, as compared to the larger 16/0 hooks, and half herring bait preferred by commercial halibut fishermen. The sex composition from the CCCFA catch east of Cape Cod were overwhelmingly female: only 27 of the 234 halibut with confirmed sex identification were male (88% female).
Age and growth
Atlantic halibut was a difficult-to-age species, even for Reader 1, who had more experience with otoliths than Reader 2. With whole otoliths, both readers agreed that they had very low confidence, and the results presented a mix of low precision and bias (Table 1). Whole otoliths yielded younger ages than sectioned otoliths across most age classes (Table 2). The opacity of the whole otoliths reduced the visibility of the annuli laid down in the first few years of life.
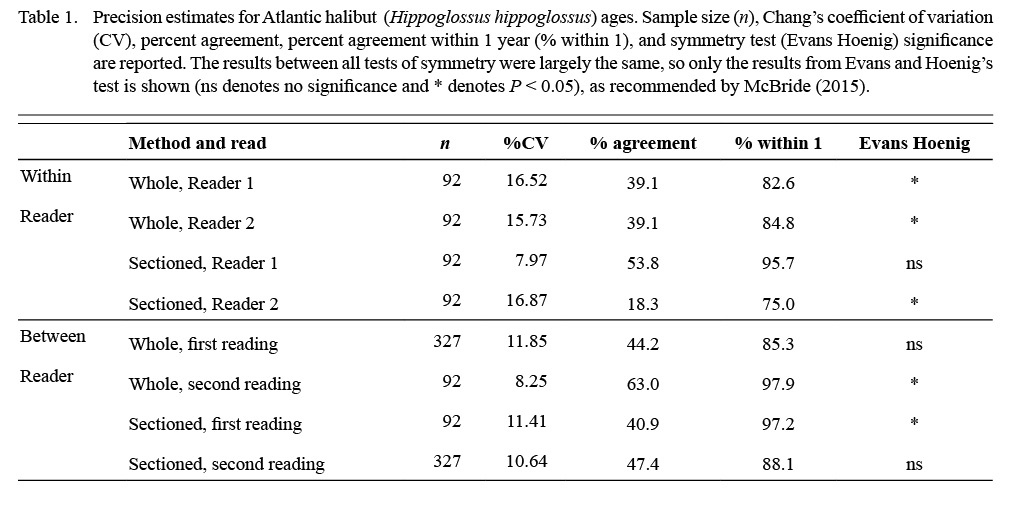
Table 1
Neither reader had specific experience ageing this species before, and precision and bias improved from the first to second reads, particularly with sectioned otoliths (Table 1; %CV, Evans Hoenig test). With sectioned otoliths, Reader 2 exhibited bias between reads where Reader 1 did not (Table 1; Evans Hoenig test). This was likely due to the experience level of the readers.
Atlantic halibut were assigned ages from 0 to 13 years. Average age of females was slightly older (5.8 years; 5.6–6.0 years) [mean; 95% confidence limits] than males (4.5; 4.0–5.0). Despite this, the maximum recorded age of males (13 years, n = 52) was slightly older than that of females (12 years, n = 247) (Fig. 6).
By gear, bottom trawls captured the largest age range (0–10 years; Fig. 6). Gillnets captured older fish but ages were truncated at the low range (4–13 years). The other gears had smaller sample sizes and the ages were within 2–11 years.
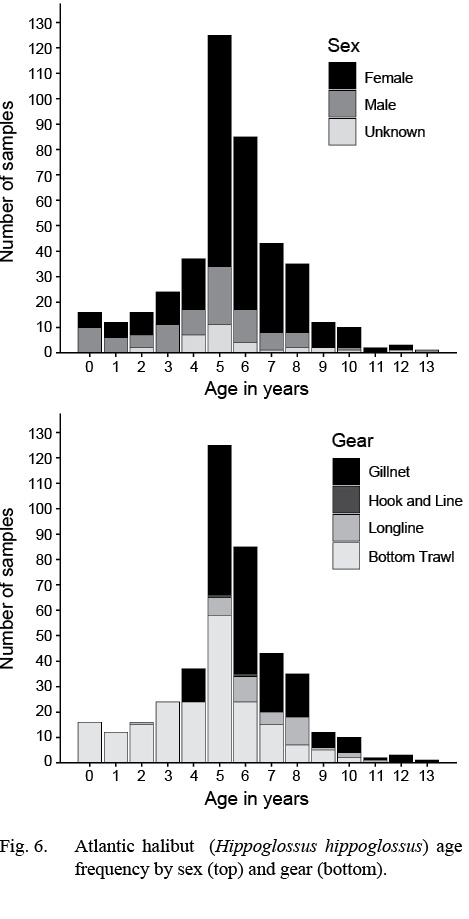
Fig. 6
Atlantic halibut demonstrated dimorphic growth between sexes. In terms of model selection, the von Bertalanffy model allowing only L∞ to vary by sex had the lowest AIC value (Table 3). Although minimal differences were seen in AIC values between the von Bertalanffy model and the Gompertz model allowing for sexual dimorphic growth, the von Bertalanffy estimated a more realistic t0 value. The final selected model for length (L, cm) at age (t, yr) was Lt = 195(1 - e -0.093(t-0.217)) for males and Lt = 214(1 - e -0.093(t-0.217)) for females (Model vB5, Table 4, Fig. 7). Females were predicted with this model to be 1.3 cm larger at age one, 6.8 cm larger at age five, 11.3 cm larger at age 10, and 14.2 cm larger at age 15 (Δ L∞ = 19 cm between sexes).
The Chapman-Robson estimate of total mortality (Z) for all data combined was 0.60 (95% confidence limit = 0.52–0.69) for ages five and older (Table 5). Estimates of Z varied by gear and sex from a low of 0.60 (0.51–0.69) for females to a high of 0.66 (0.54–0.79) for males.
Student’s t-tests revealed significant differences in length at age between gillnet captured fish and bottom trawl captured fish. Halibut aged 4 and 5 captured with a gillnet were larger than fish captured with a bottom trawl. The inverse relationship was true for ages 7 and 8.
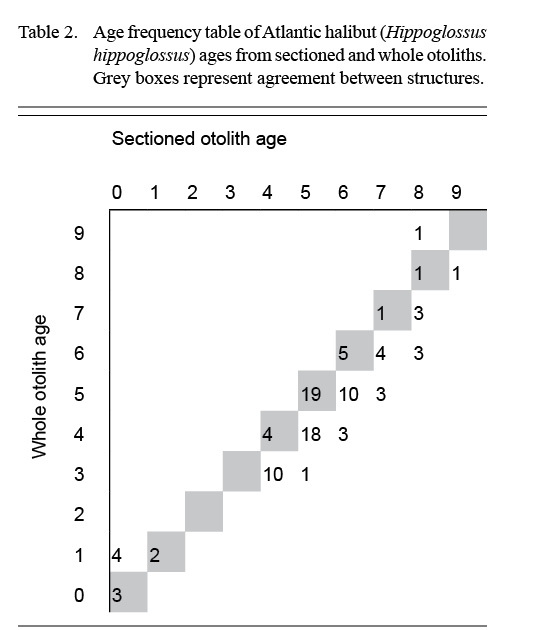
Table 2
Oogenesis
Oocyte development required several years. Primary growth (PG) oocytes were observed, as the most advanced oocyte stage, in females as young as one year old and as small as 30 cm (sample sizes, naged = 197, ntotal = 261). Further steps in oocyte development took at least four more years. Early development of cortical alveoli (EC) first appeared in females as young as five yr. and as small as 64 cm (naged = 7, ntotal = 14), and late development of cortical alveoli (LC) first appeared in females as young as six yr. and as small as 102 cm (naged = 7, ntotal = 15). First development of secondary, vitellogenic (V1) oocytes appeared in females as young as nine yr. and as small as 117 cm (naged = 1, ntotal = 6), and full development of vitellogenesis (V2) first appeared in females as young as nine yr. and as small as 81 cm (naged = 9, ntotal = 17).
An advancing cohort of vitellogenic oocytes emerged in spring and grew rapidly in the following months, creating a bimodal distribution of oocyte sizes that is characteristic of group synchronous development of vitellogenic oocytes (Table 6; Supplemental Materials). Maximum sizes of different oocyte stages were: 671 µm (LC), 828 µm (V1), 1873 µm (V2), as measured from whole oocytes (see also Supplemental Materials for oocyte diameters measured from gonad histology of individual females). The emergence and advance of a vitellogenic cohort of oocytes was also evident in monthly plots of the GSI, when the ovarian weight relative to the ovary-free female weight maximized in October at 8.5% (Fig. 8).
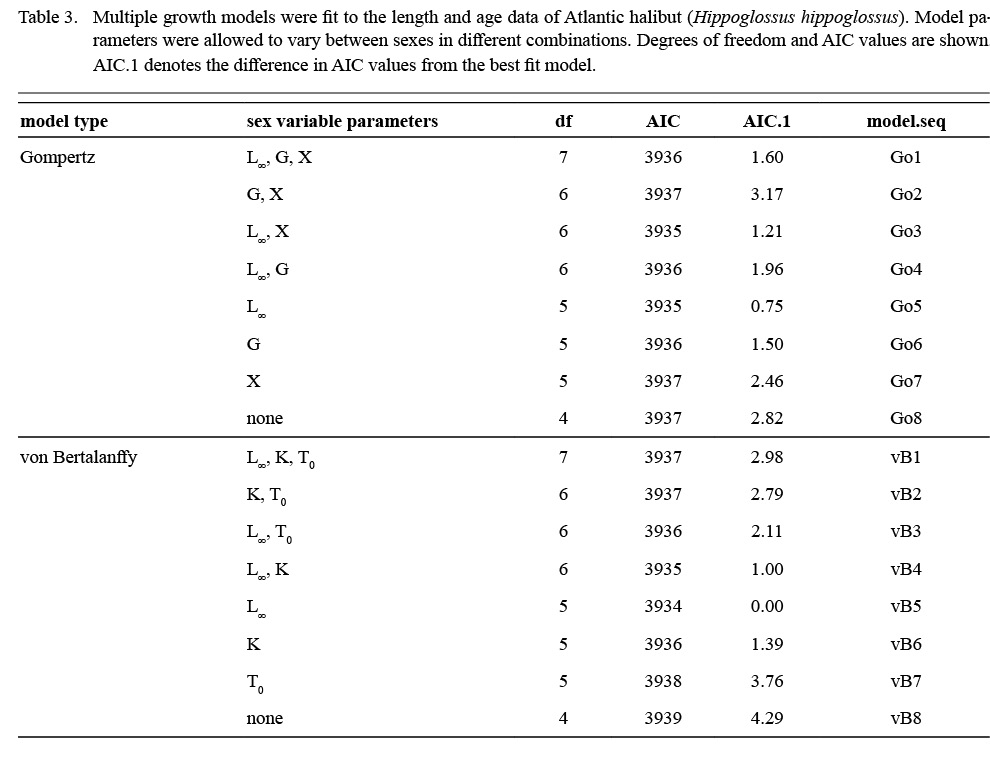
Table 3
Indications of spawning were rare and indirect. The reset of GSI values after November, suggested a likely winter spawning season (Fig. 8). No females were observed to be actively spawning, either with oocytes advanced to nucleus migration or hydration of the oocyte (Fig. 9). No females appeared in a spent condition, either with ovaries containing relatively fresh POFs, extensive atretic yolked oocytes, residual eggs in the lumen, or a mix of these traits. Only two females had any POFs at all, and while these were unambiguously recognizable as a two-layered structure with a collapsed lumen, they were well degraded and not new (Fig. 3).
Maturity and Skipping
The median length at maturity, L50, was 128 cm, with 95% confidence limits between 124 and 132 cm FL (Fig. 10). The median age at maturity, A50, was 9.6 years old, with 95% confidence limits between 9.0 and 10.8 years (Fig. 10).
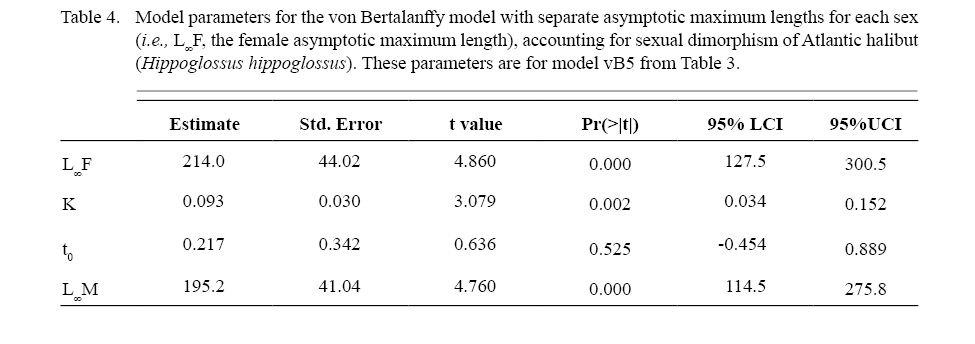
Table 4
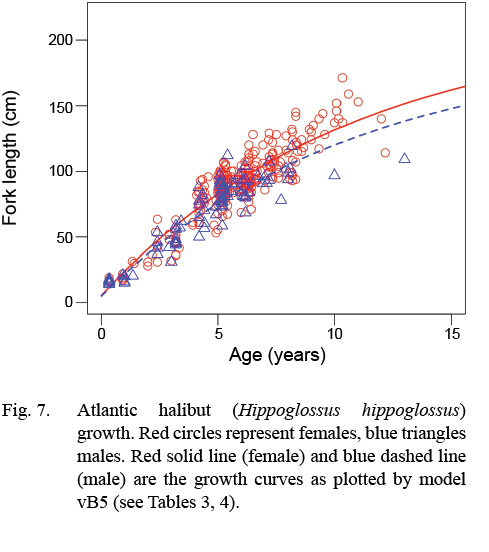
Fig. 7
Not all mature fish (i.e., with evidence that they had spawned in the past) had traits to indicate they were preparing to spawn in the next spawning season (i.e., skip spawners). One of 24 females identified as mature was large (135 cm FL), with a thick ovarian wall (1230 µ), but its most advanced oocyte stage was previtellogenic (LC). At the time it was caught, in late July, this resting fish was likely skipping that year’s spawning cycle. This sample indicates the skipping rate among mature females was 4.2%. This estimate may be biased (low) because we did not exclude any females collected in November–December, when Atlantic halibut migrate to the continental slope (see Discussion).
A second skip spawner was detected using a different selection method. Again, skipping implies that a fish has spawned in the past, so first we selected females with a thick tunica (> 500 µm). And skipping implies the spawning frequency is mis-specified (in this case every year). Finally, to check for spawning capable ovaries among fish prior to the spawning migration to continental slope waters undertaken by this species, we selected females in the month of October, which precedes the spawning period. These criteria resulted in four females, 117–145 cm FL, each with thick tunicas (685–1000 µm). Three had spawning capable ovaries: an advanced cohort of fully yolked oocytes (MAOS: V2, GSI = 5.0 – 8.5), which would predict that they would ovulate in the approaching winter spawning period. However, the smallest mature female (117 cm FL, 900 µm tunica thickness) was not prepared to spawn within a few months (MAOS: V1, GSI =0.95). This very small sample indicated the skipping rate among mature females was 25%. This estimate may be biased (high) because it assumes that the individual diagnosed as a skipper could not advance in vitellogenesis sufficiently from fall to winter to spawn, which is likely based on other northern flatfishes, but an assumption based on limited sample sizes in our study.
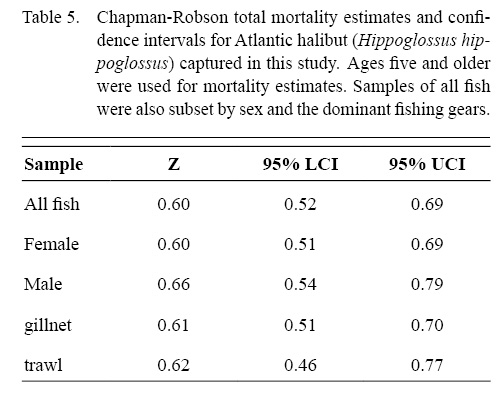
Table 5
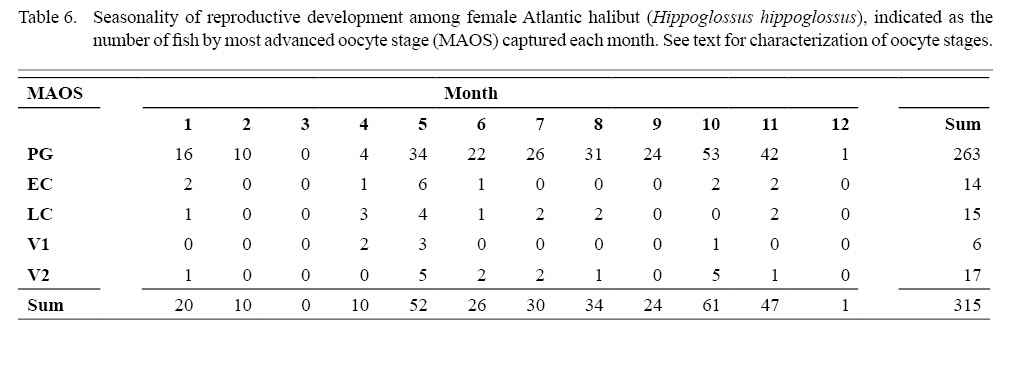
Table 6
The two skippers observed here, both aged as nine years old, were classified as mature females that are not part of the spawning stock in that reproductive cycle. One of them appeared as resting, with no evidence advancing a vitellogenic cohort of oocytes at time of capture in July, while the other female was developing, but it showed only early signs of vitellogenesis in October, with no evidence it was prepared to ovulate eggs in the winter spawning period that was imminent.
We also considered that we could identify first-time spawners, or primiparous females, based on evidence that the advance of a vitellogenic cohort of oocytes may proceed most of the year in advance of the spawning season, when in combination with immature fish having a thin tunica. One fish could be identified as a first-time spawner by these combined criteria: A female with both a thin tunica (374 µm) and a MAOS of V1 was 122 cm FL, unknown age, caught in April, with a modest gonad to body ratio (GSI = 0.96). Five of the six females with a MAOS of V1 were caught in April–May (Table 6), suggesting a pronounced seasonality initiating vitellogenesis (except for the skip spawner that initiated vitellogenesis in October, as mentioned in the preceding paragraph). This isolated example of a primiparous female was among the group of 24 mature females, representing 4.2% of the mature fish.
Changes in Proportion Mature Over Time
The proportion of fish larger than the L50 = 128 cm has increased since 2009 in both the U.S. and Canadian Scotian shelf stocks (Fig. 11). For the U.S. stock, this proportion has been increasing at a rate of approximately 18% per year (where year is a significant predictor of the odds of proportion mature, with a coefficient of 0.173 [P < 0.001], and e0.173 = 1.18 or 19%). The trend in the Canadian data is less dramatic, but based on more data (n = 6 091 lengths vs. 1 244 for the US). In the Canadian data, year predicts proportion mature with a coefficient of 0.0825 [P < 0.001], representing an annual increase in the odds of proportion mature of about 9%.
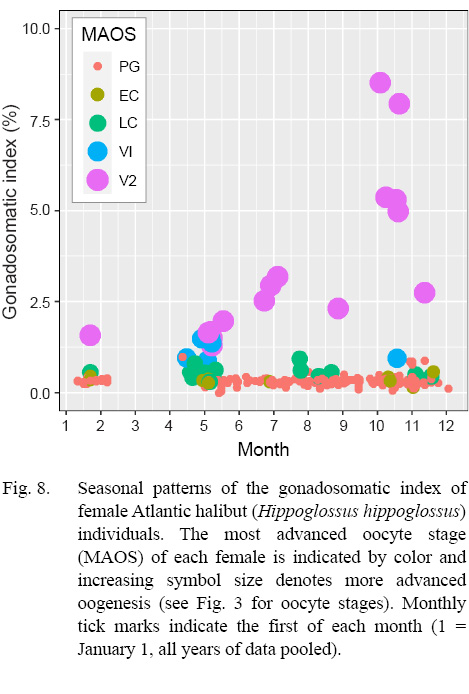
Fig. 8
Discussion
Collaborating with commercial harvesters during their operational fishing trips increased sample sizes, numbers of large fish, and spatiotemporal coverage. In terms of life history, this allowed updated, reasonably precise estimates of dimorphic growth and patterns of reproduction and maturity by Atlantic halibut in the Gulf of Maine. This study also showed an increase in the proportion of mature fish in both the U.S. and Canadian Scotian Shelf stocks, providing some hope for rebuilding the U.S. stock.
Collaborative Research
We characterize our partnership with commercial fishermen as ‘collaborative,’ rather than merely ‘cooperative,’ in that the fishermen were involved in all aspects of the project from forming the research question, to developing sampling methods, to collecting biological samples and reviewing results (Yochum et al., 2011). During the project design phase fishermen accurately projected the number of samples they as a group could deliver over two years, which increased the annual sample number from 18 per year in 2014–2016, to over 100 per year in 2017–2018. This resulted in an overall yield of 197 females, 63% of all female Atlantic halibut samples collected for the study. Our study was not designed to address the issue of highly skewed sex ratios in the commercial catch, but we note our results were very similar to the 4F:1M ratio reported in retained catches of Pacific halibut, so this does not appear to be unexpected (Loher et al., 2022). In a separate study, Hansell et al. (2020) also collaborated with Maine fishermen to develop a time series of standardized catch of Atlantic halibut, which serves as an index of abundance to track trends in population abundance of this data-limited fishery.
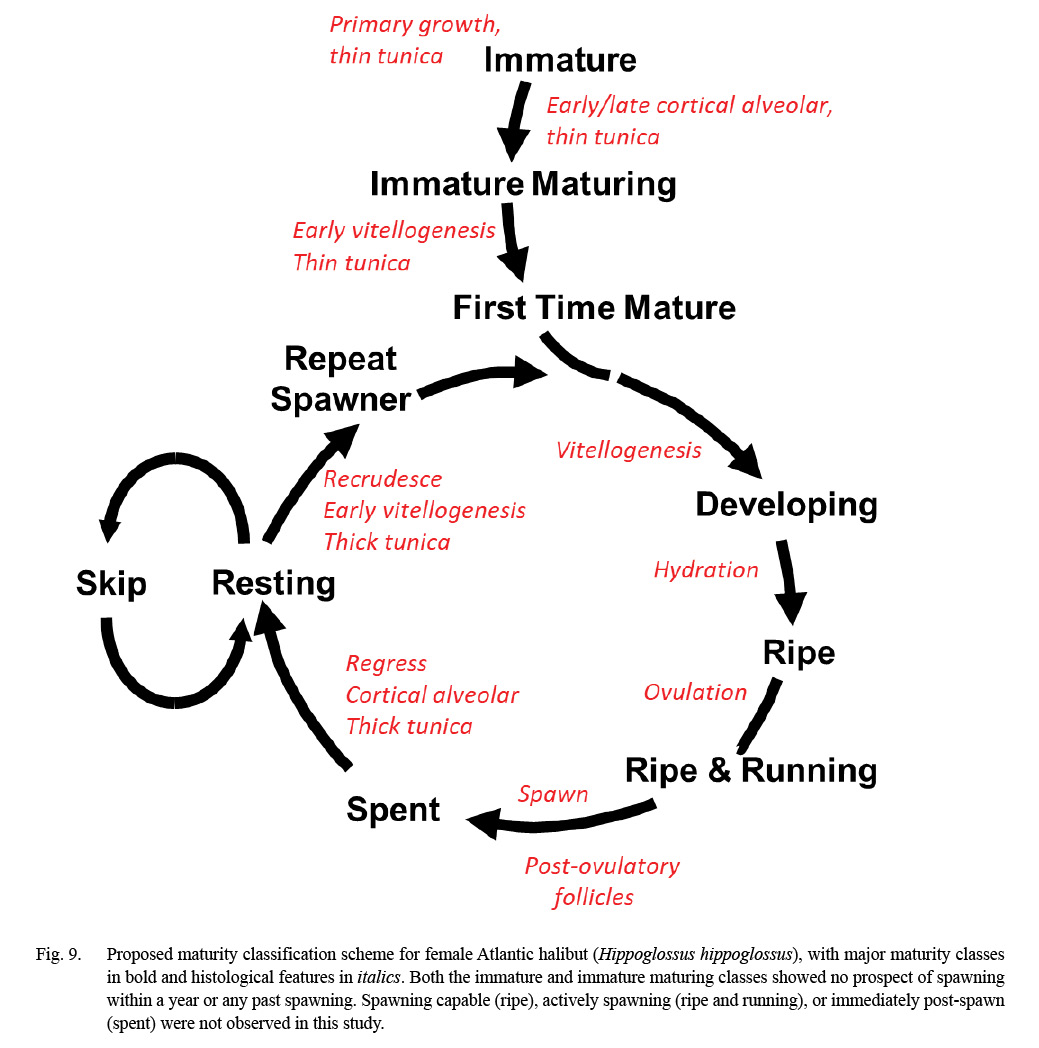
Fig. 9
Working with commercial fishermen was a highly cost-effective approach to collect biological samples for a relatively rare species like Atlantic halibut, where fishermen landed 0–5 fish per day under an exempted fishing permit. The cost of chartering fishing vessels to target Atlantic halibut would have been prohibitively expensive considering the low daily catch and absence of a targeted fishery. Through the collaborative development of the project, fishermen agreed to financial reimbursement per complete sample, which was designed to compensate fishermen for the cost of carefully completing the biological sampling and sample delivery, while at the same time not bei ng so high that fishermen would be tempted to target Atlantic halibut for the funds.
Age and growth
Atlantic halibut collected for ageing in this study were of size (14–171 cm) and age ranges (0–13 years) similar to those seen by Sigourney et al. (2006), whose samples came from a similar geographic region; in fact, many of Sigourney et al.’s samples came from the same federal bottom trawl survey which has been ongoing since the 1960s. We report ageing error rates transparently but find no comparable reports of ageing error in the literature to evaluate the relative precision in ageing this species among studies. Moreso, there are other factors that affect maximum sizes and ages in growth studies of Atlantic halibut. In particular, Armsworthy and Campana (2010) reported larger (232 cm) and older (50 years) Atlantic halibut from Canadian waters. But their larger maximum size and age may be at least partly an artifact of much larger sample size, because they examined 2 000 thin-sectioned otoliths, selected from an archive of about 65 000 fish. When sample sizes are larger, often, so is the size and age range. Also, their methods describe a post-stratification procedure of this archive (e.g., five fish per 3-cm length bin), which over represents larger and older fish relative to sampling the entire catch as we did here. Finally, the majority of their samples were from longline gear (83%), whereas the majority of our samples were from bottom trawl and gillnet gear (86%; Supplemental Materials). Longline gear catch more faster growing fish than bottom trawls (Armsworthy and Campana, 2010; Sigourney et al., 2006), whereas gillnets exhibit dome selectivity (e.g., in this study gillnets did not capture any fish younger than age four, and age four and age five fish were larger at age than those captured by bottom trawl). Other specific gear configurations (e.g., hook size on longlines) confound simple comparisons of different samples, as well. These factors, together with the rebuilt condition of Atlantic halibut in Canadian waters, all contribute to much larger and older fish in the Armsworthy and Campana (2010) report. As a historical benchmark, a halibut larger than 300 kg was caught in the Gulf of Maine in 1917 (Klein-MacPhee, 2002), whereas our largest fish, 171 cm FL, was 10 years old and weighed 44.5 kg.
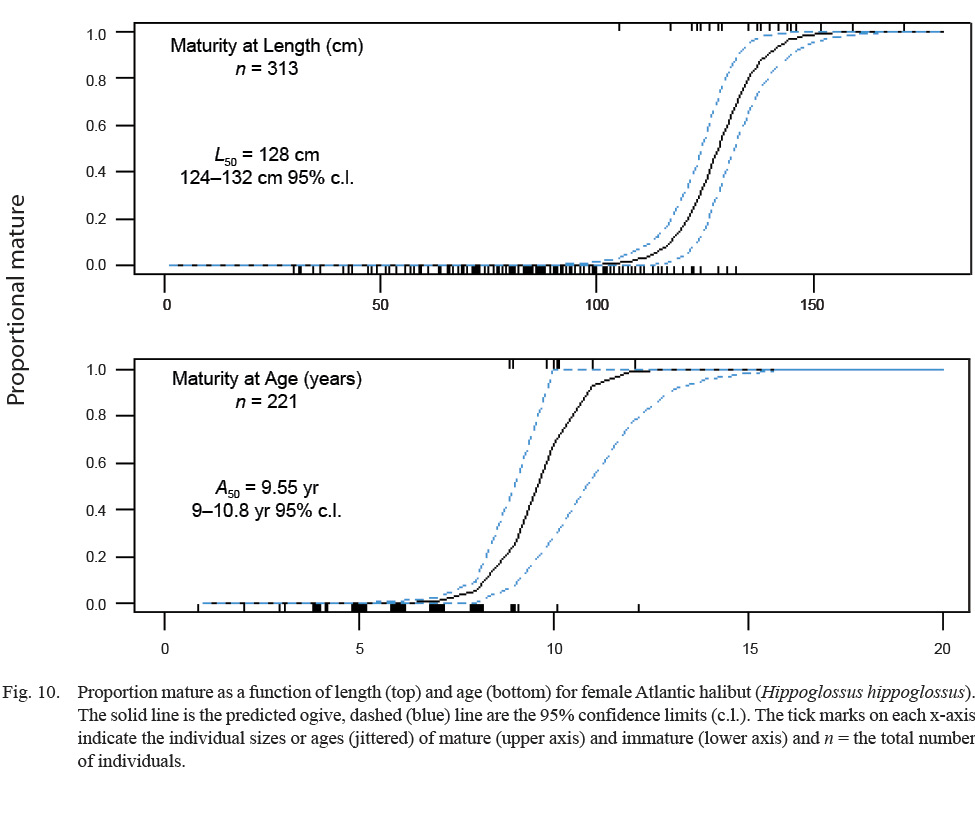
Fig. 10
Consistent with previously published work (Armsworthy and Campana, 2010; Sigourney et al., 2006; Bowering, 1986; Shackell et al., 2019), female Atlantic halibut in this study grew larger than males. The asymptotic length (L∞) parameter fit better when modeled separately by sex, with reasonable precision considering the relatively small sample size of the largest fish. Such sexual dimorphism in growth of Atlantic halibut increases the variance of size at age in unsexed samples. Our estimations of asymptotic lengths (214 cm for females and 195 cm for males) were smaller than those produced by Armsworthy and Campana (2010) (232 cm F, 175 cm M). This is not surprising given the differences in sampling design, gear configurations, and Atlantic halibut longevity in U.S. versus Canadian waters.
Given the opportunistic sampling nature of this study in both the gear used and the area sampled, mortality estimates are preliminary. The migratory nature of Atlantic halibut, coupled with the lack of very large, reproductively active individuals in our samples, suggests that our sampling efforts were not inclusive of the whole stock. The absence of those largest individuals likely biased our mortality estimates high. Two tagging studies on the Scotian Shelf and Grand Banks of the western North Atlantic Ocean showed lower total mortality estimates of Atlantic halibut: 0.37–0.46 (years: 2007–2009), or 0.24 (males, year 2009) and 0.41 (females, year 2009) (den Heyer et al., 2013; Trzcinski and Bowen, 2016). However, lower total mortality is expected in Canada, where the fishery is certified by the Marine Stewardship Council.
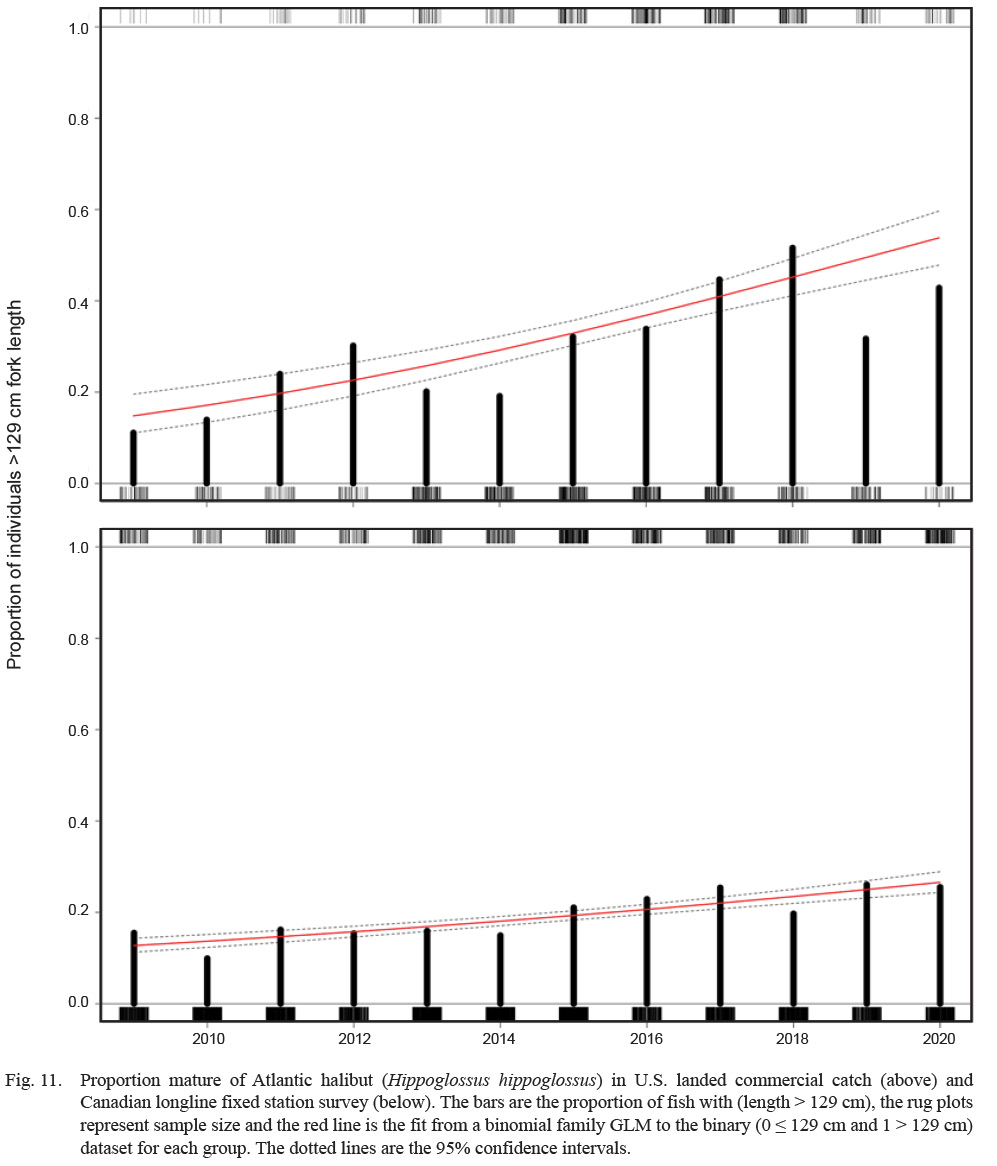
Fig. 11
Reproduction and maturity
The results from gonad histology were consistent with previous characterizations of Atlantic halibut as iteroparous with group-synchronous oocyte development with respect to vitellogenesis (Haug and Gulliksen, 1988, Neilson et al., 1993). In particular, we document a prolonged period of vitellogenesis (months), first-time maturing females (primiparity), repeat spawners (iteroparity), and new evidence of mature females skipping spawning. Fig. 9 integrates the published maturity scheme used by the Northeast Fisheries Science Center (NEFSC; Burnett et al, 1988), which is based entirely on macroscopic characters, with the processes of oogenesis and ovarian microstructure informed by gonad histology. In our region, the relative rarity of Atlantic halibut in the NEFSC bottom trawl survey is a challenge for quality assurance of training staff on at-sea maturity determinations of this species. This updated scheme (Fig. 9) will be used together with a photographic catalog of whole gonad images (see Supplemental Materials) as part of this training.
The median length at female maturity, L50 = 128 cm FL, was longer than previous reports for Atlantic halibut in the Gulf of Maine, and the median age at maturity, A50 = 9.5 years old, was older, too (Shackell et al. 2021; their Fig. 7). The most direct geographic comparison of size or age at maturity would be that of Sigourney et al. (2006), who reported female L50 = 103 (4.8 SE) cm and A50 = 7.3 (0.41 SE) yr. for Atlantic halibut from the Gulf of Maine collected during 1977–2001. A changing, in this case increasing, median size or age at maturity between Signourney et al.’s and our results could well be representative of this population as maturation schedules can change with changes in fishing pressure (Rørvik et al., 2021) and indices indicate a level or increasing population size (Hansell et al., 2020). However, as the Gulf of Maine Atlantic halibut population is data-limited, we are not able to address this directly. Older collections from fishing grounds offshore of Nova Scotia demonstrate smaller maturity: L50 = 116 (2.2 SE) cm during 1947 (McCracken, 1958) and L50 = 107 (5.2 SE) cm during 1959–1963 (Kohler, 1967). McCracken (1958) estimated a similar age at maturity, at roughly 10–12 years. The several decade differences between some of these studies, and an expectation that maturation is not time-invariant, confounds simple geographic comparisons (but see Shackell et al. 2021 who assemble such a comparison).
The differences in maturity schedules are also likely the result of our use of ovarian histology, whereas previous reports used macroscopic methods. Our ability to be more precise about the presence of vitellogenesis, likely increases the estimate of age at maturity by one year. Oogenesis proceeded slowly in Atlantic halibut, with only pre-vitellogenic growth stages observed among females < 9 years old in our samples. Vitellogenesis unfolded over most of the year preceding spawning, much like shown for Pacific halibut by Fish et al. (2022). We postulate this creates a situation, when the ovary is viewed macroscopically, that some fish with MAOS = LC are confused as resting and erroneously classified as mature, as the case for other regional flatfish such as winter flounder (McBride et al., 2013). Using gonad histology, where the tunica thickness can be accurately determined, can distinguish immature and mature individuals. Given the potential for measurement bias between histological and macroscopic studies, a conservative conclusion is that the mean age and size at maturity of female Atlantic halibut in the Gulf of Maine and around Nova Scotia has been fairly stable at 7–12 years and 100–130 cm.
Details of oocyte maturation and ovulation were not observed directly, presumably because Atlantic halibut migrate to the continental slope, outside of our sampling range, to spawn. Liu et al. (2019) observed tagged halibut in the Gulf of Maine making long-distance movements suggesting a spawning migration. These fish move to the Northeast Channel off Georges Bank, where neither fishing nor fishery-independent surveys takes place, explaining why we did not collect spawning halibut. Liu et al. did not observe abrupt ascents, which could be interpreted as spawning activity, but halibut were in these deep, presumptive spawning habitats during winter, from January to May.
Further east, in the Gulf of St. Lawrence, spawning occurred at depths of 200 m or deeper during winter, from mid-December to late-April with a peak in mid-February (Le Bris et al., 2018; Gatti et al., 2020). Still further east, in deep (800–1000 m) waters south of the Grand Banks, Armsworthy et al. (2014) report abrupt ascents, interpreted as spawning rises, during later autumn – early winter, from October to January. In the eastern Atlantic, the spawning season is so predictable (December–March) that the halibut gillnet fishery in Norway has been prohibited during parts of January–February since the 1950s (Godø and Haug, 1988; Haug and Gulliksen, 1988).
On the other American coast, Pacific halibut have been collected on their spawning grounds, with multiple lines of evidence of winter spawning seasonality (January–February). Evidence of spawning includes the appearance of POFs, and a more complete characterization of oogenesis and gonad maturation (e.g., maximum oocyte size = 2.0 –2.5 mm, a maximum reported GSI of 15% for females; Fish et al., 2020, 2022).
Presumably, mature U.S. Atlantic halibut females that remain in our sampling range during the spawning season are skip spawners, but this requires additional, precise information about their spawning migration and an ability to capture them across these habitats (Rodgveller et al. 2016). Instead, we interrogated two samples for which we considered immature and mature females should be well mixed. Both skip spawners detected were, not surprisingly, young (9-year-old fish). Skip spawning is more likely among smaller and younger fish that may be energetically challenged compared to larger and more experienced females (Burton, 1994; Rideout and Tomkiewicz, 2011; Tronbøl et al., 2022). The calculations presented herein assume a prolonged period of vitellogenesis, which appears supported by our data, and a winter spawning period, which is consistently demonstrated by a number of studies reviewed in the previous paragraphs. Both samples used for these calculations were small, and therefore preliminary, but of interest as the first estimates for this species.
Accuracy in skip spawning rates is important for correctly specifying the spawning stock biomass in stock assessments, when not all mature fish spawn as expected. Electronic tagging data is a complementary tool that could contribute to evaluating skip spawning rates for halibut. Tagging data for Pacific halibut suggest that approximately 10% of mature fish are skip spawning (Loher and Seitz, 2008). The number of fish tagged and released in our region is increasing, which should soon reveal more about Atlantic halibut spawning times, frequencies (e.g., skipping), and the behaviors of spawning contingents in U.S. and neighboring waters (W. DeVoe, State of Maine, pers. comm.). Multiple lines of evidence about skip spawning are needed for robust estimates of skipping rates in different populations of halibut species.
Management Implications
The U.S. Atlantic halibut stock has been depleted for at least a century (Grasso, 2008). Recent U.S. stock assessments have shown evidence of modest population increases over the last decade, and as evident herein, the proportion of mature fish in both U.S. and Canada waters has increased over the same period. These coupled trends are an indicator of an increase in reproductive potential. Now that an estimate of size at maturity exists, it is reasonably cost effective to continue to track the proportion of fish above this size as a proxy for mature females, which could be a useful qualitative assessment tool for this data-limited stock. However, it is important to understand the fishery context for using such an approach because alternative interpretations exist. For example, an increase of proportion mature in an increasing stock is contra-indicative of high fishing pressure, whereas an increase of proportion mature in a stable or decreasing stock may be indicative of recent recruitment failure.
Comparing the proportion mature between spatially adjacent stocks may be of some interest as well. In this case, we compared the proportion mature in U.S. waters to the Canadian Scotian Shelf population, within the Gulf of Maine (Fig. 1). Proportion mature is increasing in both areas, although the trend is less defined in the Canadian data. Several factors may drive the differences in magnitude of trend between the areas. The Canadian data are survey based, while the U.S. data are from commercially landed fish. Current U.S. regulations stipulate that only one Atlantic halibut per trip can be landed, which equates to a tendency for the largest fish encountered to be landed and all others discarded. The Canadian data are measurements of all fish encountered on the survey. The selectivities associated with the two data sets are therefore quite different and may influence results. The Canadian stock has been stable or increasing for many years and supports a large and valuable fishery. The U.S. stock has been depleted for a century and has not supported a directed fishery for most of that period. Therefore, the scope for stock increase (i.e. the distance from theoretical carrying capacity) in the U.S. is probably much greater. Finally, the extent of movement between the two areas is unknown and it is possible that some mature fish from Canada have relocated to U.S. waters, which might potentially affect the proportion mature in each area.
Alternatively, size composition of discarded fish from U.S. fishery observer data could be used to compare the U.S. and Canadian stocks. Unfortunately, Atlantic halibut encounter rates in the United States remain low, observer coverage of longline gear (the gear most likely to capture large Atlantic halibut) is low (generally < 10%; Hogan et al., 2019), and there are very few mature fish among the discarded Atlantic halibut length data over the time period concerned (n = 6 fish). There is some possibility of using the kept fish from U.S. observer data, but there are far more Atlantic halibut observations in the landings data and combining the two introduces the possibility of double counting. Ideally, the U.S. data would come from a fishery independent survey, as the Canadian data does –which removes the possibility of spatial bias commonly associated with fishery dependent data (Paloheimo and Dickie, 1964) – but U.S. surveys do not sample Atlantic halibut well (Hennen, 2015).
A truncated age distribution is a common symptom of a stock in poor condition (Hilborn and Peterman, 1996). This describes a situation in which a disproportionate amount of the mature fish have been removed, leaving mostly young and immature fish in the population. This situation is dire for any stock, as mature fish supply the reproductive material for future recruitment. In this sense, a high proportion of mature fish might indicate a healthy stock. When a fishery targets immature fish, they can be removed before reproducing, which can reduce reproductive potential, as for example, when a large year class of fish is fished down before reaching maturity. When a fishery targets mature fish, the effect depends on the contribution of each age class to reproductive value (Kindvater et al., 2016; Marshall et al., 2021). Overall, we are cautious about simple interpretations of proportion mature as an indicator of stock health. Nonetheless, an increasing trend of proportion mature together with an increasing trend of stock size is generally an indicator of a healthy stock and relatively low mortality. As there is evidence that both the U.S. and Scotian shelf Atlantic halibut stocks are increasing (Rago, 2018; DFO, 2020), our new observations of an increase of proportions mature indicates an increase in the reproductive potential of each stock.
Acknowledgements
We begin by thanking our collaborative fishing partners, particularly harvesters working with the Cape Cod Commercial Fishermen’s Alliance (CCCFA; B. Eldridge, B. Hopkins, B. McCann, J. Amaru, W. Amaru, N. Muto, E. Hesse, J. Nash, G. Connors and G. Walinski) who conceptualized and helped design this project and provided the majority of halibut samples, as well as others in Maine and those involved in the Northeast Fisheries Science Center’s (NEFSC) study fleet. We also appreciate the efforts of all hands participating in fishery-independent sampling, who produced a steady stream of samples from the NEFSC’s bottom trawl survey and bottom longline survey, as well as samples by surveys operated by the State of Maine and Massachusetts’ Division of Marine Fisheries (MDMF). Specific individuals that facilitated collecting from these sources included: G. Gianesin, W. Hoffman, M. Kersula C. Bank, D. McElroy, and D. St. Amand. We also thank the following: G. Skomal, W. Hoffman (MDMF) for fieldwork logistics; E. Koob, C. Draghetti (MDMF) for contributions to ageing; R. Marshall (The Nature Conservancy [TNC]/CCCFA) for designing and administering the commercial sampling scheme; Melissa Sanderson (CCCFA) for supporting shoreside sampling and logistics; M. Wuenschel (NEFSC) for contributions to the maturity work; S. Talmage NOAA’s Greater Atlantic Regional Fisheries Office) for facilitating fishing access to the commercial fleet; J. Akin (TNC) for administering this award from NOAA. This project was funded by NOAA Fisheries’ Saltonstall-Kennedy Grant Program (Award 16GAR057 to TNC) with additional funding and in-kind match from TNC.
References
Armsworthy, S. L., and Campana, S. E. 2010. Age determination, bomb-radiocarbon validation and growth of Atlantic halibut (Hippoglossus hippoglossus) from the Northwest Atlantic. Environmental Biology of Fishes, 89: 279–295. https://link.springer.com/article/10.1007/s10641-010-9696-8.
Armsworthy, S. L., Trzcinski, M. K., and Campana, S. E. 2014. Movements, environmental associations, and presumed spawning locations of Atlantic halibut (Hippoglossus hippoglossus) in the northwest Atlantic determined using archival satellite pop-up tags. Marine Biology, 161: 645–656. https://doi.org/10.1007/s00227-013-2367-5
Barnett, L. A. K., Branch, T. A., Ranasinghe, R. A., and Essington, T. E. 2017. Old-growth fishes become scarce under fishing. Current Biology, 27: 2843–2848. https://doi.org/10.1016/j.cub.2017.07.069.
Blaylock, J., and Legault, C. M. 2012. Atlantic halibut. In Assessment or Data Updates of 13 Northeast Groundfish Stocks through 2010. Northeast Fisheries Science Center Reference Document 12-06, https://repository.library.noaa.gov/view/noaa/4060.
Bowering, W. R. 1986. The distribution, age, growth and sexual maturity of Atlantic halibut (Hippoglossus hippoglossus) in the Newfoundland and Labrador area of the northwest Atlantic. Canadian Fisheries and Aquatic Sciences Technical Report No 1432: 34. http://publications.gc.ca/site/eng/456903/publication.html.
Brown, N. P., Shields, R. J., and Bromage, N. R. 2006. The influence of water temperature on spawning patterns and egg quality in the Atlantic halibut (Hippoglossus hippoglossus L.). Aquaculture, 261(3): 993–1002. https://doi.org/10.1016/j.aquaculture.2006.08.025
Burnett, J., O’Brien, L., Mayo, R. K., Darde, J. A., and Bohan, M. 1989. Finfish maturity sampling and classification schemes used during Northeast Fisheries Center bottom trawl surveys, 1963–89. Woods Hole (MA): NOAA Technical Memorandum NMFS-F/NEC-76: 1–14. https://repository.library.noaa.gov/view/noaa/5951
Burton, M. P. M. 1994. A critical period for nutritional control of early gametogenesis in female winter flounder, Pseudopleuronectes americanus (Pisces: Teleostei). Journal of Zoology, 233: 405–415. https://doi.org/10.1111/J.1469-7998.1994.TB05273.X.
CCCFA [Cape Cod Commercial Fishermen’s Alliance]. 2018. Halibut Biological Sampling. https://www.youtube.com/watch?v=XhMV9e3UA14
Chang, W. Y. B. 1982. A statistical method for evaluating the reproducibility of age determination. Canadian Journal of Fisheries and Aquatic Sciences, 39: 1208–1210. https://doi.org/10.1139/f82-158.
DFO, (Department of Fisheries and Oceans, Canada). 2020. Stock status update of Atlantic halibut (Hippoglossus hippoglossus) on the Scotian Shelf and Southern Grand Banks in NAFO Divisions 3NOPs4VWX5Zc. DFO Canadian Science Advisory Secretariat, Science Response. https://waves-vagues.dfo-mpo.gc.ca/Library/40884454.pdf.
Fairclough, D. V., Brown, J. I., Carlish, B. J., Crisafulli, B. M., and Keay, I. S. 2014. Breathing life into fisheries stock assessments with citizen science. Scientific Reports, 4: 7249. https://doi.org/10.1038/srep07249.
Fish, T., Wolf, N., Harris, B. P., and Planas, J. V. 2020. A comprehensive description of oocyte developmental stages in Pacific halibut, Hippoglossus stenolepis. Journal of Fish Biology, 97: 1880–1885. https://onlinelibrary.wiley.com/doi/abs/10.1111/jfb.14551.
Fish, T. M., Wolf, N., Smeltz, T. S., Harris, B. P., and Planas, J. V. 2022 accepted. Reproductive biology of female Pacific halibut (Hippoglossus stenolepis) in the Gulf of Alaska. Frontiers in Marine Science. https://doi.org/10.3389/fmars.2022.801759
Gatti, P., Robert, D., Fisher, J. A. D., Marshall, R. C., and Le Bris, A. 2020. Stock-scale electronic tracking of Atlantic halibut reveals summer site fidelity and winter mixing on common spawning grounds. ICES Journal of Marine Science, https://doi.org/10.1093/icesjms/fsaa162
Grasso, G. M. 2008. What appeared limitless plenty: The rise and fall of the nineteenth-century Atlantic halibut fishery. Environmental History, 13: 66–91. www.jstor.org/stable/25473194.
Godø, O. R,, and Haug, T. 1988. Tagging and recapture of Atlantic Halibut (Hippoglossus hippoglossus) in Norwegian waters. ICES Journal of Marine Science, 44(2):169–179. https://doi.org/10.1093/icesjms/44.2.169
Hansell, A. C., DeCelles, G. R., Kersula, M. E., and Cadrin, S. X. 2020. Incorporating harvesters’ knowledge into an index of abundance for Atlantic halibut in the northwest Atlantic. Transactions of the American Fisheries Society, 149: 741–752. https://doi.org/10.1002/tafs.10268.
Haug, T., and Gulliksen, B. 1988. Fecundity and oocyte sizes in ovaries of female Atlantic halibut, Hippoglossus hippoglossus (L). Sarsia, 73(4):259–261. https://doi.org/10.1080/00364827.1988.10413411
Hennen, D. 2015. Atlantic halibut. In Operational Assessment of 20 Northeast Groundfish Stocks, Updated Through 2014. Northeast Fisheries Science Center Ref Doc 15-24, https://repository.library.noaa.gov/view/noaa/5293.
2020. Atlantic halibut. In Operational Assessment of 14 Northeast Groundfish Stocks, Updated through 2018. Northeast Fisheries Science Center Reference Document, Prepublication document, https://s3.amazonaws.com/nefmc.org/8_Prepublication-NE-GrndfshOp-Assessment-1-7-2020-revision.pdf.
Hilborn, R. M., and Peterman, R. M. 1996. The development of scientific advice with incomplete information in the context of the Precautionary Approach. FAO Fisheries Technical Paper. http://www.fao.org/3/w1238e/W1238E04.htm.
den Heyer, C. E., Schwarz, C. J., and Trzcinski, M. K. 2013. Fishing and natural mortality rates of Atlantic halibut estimated from multiyear tagging and life history. Transactions of the American Fisheries Society, 142: 690–702. https://doi.org/10.1080/00028487.2012.760482.
Hixon, M. A., Johnson, D. W., and Sogard, S. M. 2014. BOFFFFs: on the importance of conserving old-growth age structure in fishery populations. ICES Journal of Marine Science: Journal du Conseil, 71: 2171–2185. https://doi.org/10.1093/icesjms/fst200.
Hovgård, H., and Riget, F. F. 1992. Comparison of longline and trawl selectivity in cod surveys off West Greenland. Fisheries Research, 13: 323–333. https://doi.org/10.1016/0165-7836(92)90085-8.
Karlson, S., Michalsen, K., and Folkvord, A. 2013. Age determination of Atlantic halibut (Hippoglossus hippoglossus L.) along the coast of Norway: status and improvements. ICES Journal of Marine Science: Journal du Conseil, 70: 50–55. http://doi.org/10.1093/icesjms/fss174.
Kennedy, J., Gundersen, A. C., Høines, A. S., and Kjesbu, O. S. 2011. Greenland halibut (Reinhardtius hippoglossoides) spawn annually but successive cohorts of oocytes develop over 2 years, complicating correct assessment of maturity. Canadian Journal of Fisheries and Aquatic Sciences, 68: 201–209. https://doi.org/10.1139/F10-149.
Kersula, M., and Seitz, A. 2019. Diverse migratory behaviors of Atlantic halibut (Hippoglossus hippoglossus, L.) based on the 2000–2017 Maine halibut tagging program. Journal of Northwest Atlantic Fishery Science, 49: 13–24. https://doi.org/10.2960/J.v50.m719.
Kess, T., Einfeldt A. L., Wringe, B., Lehnert, S. J., Layton, K. K. S., McBride, M. C., Robert, D., Fisher ,J., Le Bris ,A., den Heyer, C., Shackell, N., Ruzzante, D. E., Bentzen, P., and Bradbury, I. R. 2021. A putative structural variant and environmental variation associated with genomic divergence across the Northwest Atlantic in Atlantic Halibut. ICES Journal of Marine Science, 78: 2371–2384. https://doi.org/10.1093/icesjms/fsab061.
Kindsvater, H. K., Mangel, M., Reynolds, J. D., and Dulvy, N. K. 2016. Ten principles from evolutionary ecology essential for effective marine conservation. Ecology and Evolution, 6: 2125–2138. https://doi.org/10.1002/ece3.2012.
Klein-MacPhee, G. 2002. Righteye flounders. Family Pleuronectidae. In B.B. Collette and G. Klein-MacPhee (Eds.), Bigelow and Schroeder’s Fishes of the Gulf of Maine, 3rd ed. Smithsonian Institution Press. pp. 560–587.
Kohler, A. C. 1967. Size at maturity, spawning season, and food of Atlantic halibut. Journal of the Fisheries Research Board of Canada, 24: 53–66. https://doi.org/10.1139/f67-006.
Le Bris, A., Fisher, J. A. D., Murphy, H. M., Galbraith, P. S., Castonguay, M., Loher T., and Robert ,D. 2018. Migration patterns and putative spawning habitats of Atlantic halibut (Hippoglossus hippoglossus) in the Gulf of St. Lawrence revealed by geolocation of pop-up satellite archival tags. ICES Journal of Marine Science, 75: 135–147. https://doi.org/10.1093/icesjms/fsx098
Liu, C., Bank, C., Kersula, M., Cowles, G. W., Zemeckis, D. R., Cadrin, S. X., and McGuire, C. 2019. Movements of Atlantic halibut in the Gulf of Maine based on geolocation. ICES Journal of Marine Science, 76: 2020–2032. https://doi.org/10.1093/icesjms/fsz169.
Loher, T., McCarthy, O., Sadorus, L. L., Erikson, L. M., Simeon, A., Drinan, D. P., Hauser, L., Planas, J. V., and Stewart, I. J. 2022. A Test of Deriving Sex-Composition Data for the Directed Pacific Halibut Fishery via At-Sea Marking. Marine and Coastal Fisheries 14(4): e10218. https://doi.org/10.1002/mcf2.10218
Loher, T., and Seitz, A. C. 2008. Characterization of active spawning season and depth for eastern Pacific halibut (Hippoglossus stenolepis), and evidence of probable skipped spawning. Journal of Northwest Atlantic Fishery Science, 41: 23–36. https://doi.org/10.2960/J.v41.m617.
Marshall, D., Bode, M., Mangel, M., Arlinghaus, R., and Dick, E. 2021. Reproductive hyperallometry and managing the world’s fisheries. Proceedings of the National Academy of Sciences, 118: e2100695118. https://doi.org/10.1073/pnas.2100695118.
McBride, R. S. 2015. Diagnosis of paired age agreement: a simulation of accuracy and precision effects. ICES Journal of Marine Science: Journal du Conseil, 72: 2149–2167. https://doi.org/10.1093/icesjms/fsv047.
McBride, R. S., Wuenschel, M. J., Nitschke, P., Thornton, G., and King, J. R. 2013. Latitudinal and stock-specific variation in size- and age-at-maturity of female winter flounder, Pseudopleuronectes americanus, as determined with gonad histology. Journal of Sea Research, 75: 41–51. https://doi.org/10.1016/j.seares.2012.04.005.
McCracken, F. D. 1958. On the biology and fishery of the Canadian Atlantic halibut, Hippoglossus hippoglossus L. Journal of the Fisheries Research Board of Canada, 15: 1269–1311. http://dx.doi.org/10.1139/f58-070.
McElroy, W. D., O’Brien, L., Blaylock, J., Martin, M. H., Rago, P. J., Hoey, J. J., and Sheremet, V. A. 2019. Design, Implementation, and Results of a Cooperative Research Gulf of Maine Longline Survey, 2014–2017. NOAA Technical Memorandum NMFS-NE-249. https://doi.org/10.25923/2sgn-mx62.
Neilson, J. D., Kearney, J. F., Perley, P., and Sampson, H. 1993. Reproductive biology of Atlantic halibut (Hippoglossus hippoglossus) in Canadian waters. Canadian Journal of Fisheries and Aquatic Sciences, 50: 551–563. http://www.nrcresearchpress.com/doi/abs/10.1139/f93-064#.V45zEjFvk1s.
Norberg, B., Valkner, V., Huse, J., Karlsen, I., and Grung, G. L. 1991. Ovulatory Rhythms and Egg Viability in the Atlantic Halibut (Hippoglossus hippoglossus). Aquaculture, 97(4): 365–371. https://doi.org/10.1016/0044-8486(91)90328-5
Ogle, D. H., Wheeler, P., and Dinno, A. 2020. FSA, version 0.8.30, https://github.com/droglenc/FSA.
Paloheimo, J. E., and Dickie, L. M. 1964. Abundance and fishing success. Rapports et Procès-Verbaux des Réunions du Conseil International pour l’Exploration de la Mer, 155: 152–163. https://www.ices.dk/sites/pub/Publication%20Reports/Marine%20Science%20Symposia/Phase%202/Rapport%20et%20Proces-Verbaux%20des%20Reunions%20-%20Volume%20155%20-%201964%20-%20Partie%2031%20de%2043.pdf.
Politis, P. J., Galbraith, J. K., Kostovick, P., and Brown, R. W. 2014. Northeast Fisheries Science Center Bottom Trawl Survey Protocols for the NOAA Ship Henry B. Bigelow. Northeast Fisheries Science Center Reference Document 14-06: [Accessed February 2021]. https://repository.library.noaa.gov/view/noaa/4825.
Press, Y. K., McBride, R. S., and Wuenschel, M. J.2014. Time course of oocyte development in winter flounder (Pseudopleuronectes americanus) and spawning seasonality for the Gulf of Maine, Georges Bank, and Southern New England stocks. Journal of Fish Biology, 85: 421–445. http://onlinelibrary.wiley.com/doi/10.1111/jfb.12431/full.
Rago, P. J. 2018. Halibut Assessment Report for 2017. Prepared for the New England Fishery Management Council. http://s3.amazonaws.com/nefmc.org/Halibut–Assessment–Report–draft–12–01–2017.pdf.
Read, A. N., and Hartley, T. W., (Editors). 2006. Partnerships for a common purpose: Cooperative fisheries research and management. Bethesda, MD: American Fisheries Society. https://doi.org/10.47886/9781888569858.ch31
Rideout, R. M., and Tomkiewicz, J. 2011. Skipped spawning in fishes: more common than you might think. Marine and Coastal Fisheries, 3: 176–189. http://www.tandfonline.com/doi/abs/10.1080/19425120.2011.556943.
Rodgveller, C., Stark, J., Echave, K., and Hulson, P. 2016. Age at maturity, skipped spawning, and fecundity of female sablefish (Anoplopoma fimbria) during the spawning season. Fishery Bulletin, 114: 89–102. https://doi.org/10.7755/FB.114.1.8.
Rørvik, C, Bogstad, B, Ottersen, G, and Kjesbu, O. 2021. Long-term interplay between harvest regimes and biophysical conditions may lead to persistent changes in age-at-sexual maturity of Northeast Arctic cod (Gadus morhua). Canadian Journal of Fisheries and Aquatic Sciences, 79: 576–586. https://doi.org/10.1139/cjfas-2021-0068
Seitz, A. C., Evans, M. D., Courtney, M. B., and Kanwit, J. K. 2016. Continental shelf residency by adult Atlantic halibut electronic tagged in the Gulf of Maine. Journal of Northwest Atlantic Fishery Science, 48: 33–40. https://doi.org/10.2960/J.v48.m713.
Shackell, N. L., Ferguson, K. J., denHeyer, C. E., Brickman, D., Wang, Z., and Ransier, K. T. 2019. Growing degree-day influences growth rate and length of maturity of Northwest Atlantic halibut (Hippoglossus hippoglossus L.) across the southern stock domain. Journal of Northwest Atlantic Fishery Science, 50: 25–35. https://doi.org/10.2960/J.v50.m716.
Shackell, N. L., Fisher, J. A. D., den Heyer, C. E., Hennen, D. R., Seitz, A. C., Le Bris, A., Robert, D., Kersula, M. E., Cadrin, S. X., McBride, R. S., McGuire, C. H., Kess, T., Ransier, K. T., Liu, C., Czich, A., and Frank, K. T. 2021. Spatial ecology of Atlantic halibut across the northwest Atlantic: A recovering species in an era of climate change. Reviews in Fisheries Science & Aquaculture: 1–25. https://doi.org/10.1080/23308249.2021.1948502.
Shackell, N. L., Frank, K. T., Nye, J. A., and den Heyer, C. E. 2016. A transboundary dilemma: dichotomous designations of Atlantic halibut status in the Northwest Atlantic. ICES Journal of Marine Science: Journal du Conseil. https://doi.org/10.1093/icesjms/fsw042.
Sigourney, D. B., Ross, M. R., Brodziak, J., and Burnett, J. 2006. Length at age, sexual maturity and distribution of Atlantic halibut, Hippoglossus hippoglossus L., off the Northeast USA. Journal of Northwest Atlantic Fishery Science, 36: 81–90. https://doi.org/10.2960/J.v36.m574.
Thiel, M., Penna-Diaz, M. A., Luna-Jorquera, G., Salas, S., Sellanes, J., and Stotz, W. 2014. Citizen scientists and marine research: volunteer participants, their contributions, and projection for the future. In R.N. Hughes, D.J. Hughes, and I.P. Smith (Eds.) Oceanography and Marine Biology: An Annual Review, Vol 52: CRC Press-Taylor and Francis Group. pp: 257–314. https://doi.org/10.1201/b17143-6
Torrejon-Magallanes, J. 2020. https://github.com/ejosymart/sizeMat.
Tronbøl, F., Johannesen, E., Alix, M., dos Santos Schmidt, T. C., Charitonidou, K., Folkvord, A., and Kjesbu, O. S. 2022. Tracking oocyte development and the timing of skipped spawning for north-east Arctic haddock (Melanogrammus aeglefinus). Journal of Fish Biology 100(6): 1464–1474. https://doi.org/10.1111/jfb.15057
Trzcinski, M. K., and Bowen, W. D. 2016. The recovery of Atlantic halibut: a large, long-lived, and exploited marine predator. ICES Journal of Marine Science: Journal du Conseil, 73: 1104–1114. https://doi.org/10.1093/icesjms/fsv266.
Yochum, N., Starr, R. M., and Wendt, D. E. 2011. Utilizing fishermen knowledge and expertise: Keys to success for collaborative fisheries research. Fisheries, 36: 593–605.
Zwanenburg, K., Wilson, S., Branton, R., and Brien, P. 2003. Halibut on the Scotian Shelf and the Southern Grand Banks – Current estimates of population status. Bedford Institute of Oceanography, Research Document 2003/046, Dartmouth, NS: 34 pp.
Citation: McBride, R.S., Maynard, G.A., Elzey, S.P., Hennen, D.R., Tholke, E.K. Runnebaum, J.M. and McGuire, C.H. 2022. Evaluating growth dimorphism, maturation, and skip spawning of Atlantic halibut in the Gulf of Maine using a collaborative research approach. J. Northw. Atl. Fish. Sci., 53: 57–77. https://doi.org/10.2960/J.v53.m736