Introduction
Parents invest in offspring quality in many ways; spanning from mate choice, through careful selection of nursery areas, to parental care. Parental investment towards offspring quality is typically traded off against their own survival and future reproduction (Williams, 1966). For many species, the ideal nursery areas for their offspring may often be less profitable or even completely unsuitable habitats for the parents. A vast number of animals, both on land and in the sea, are capable of performing astonishing migrations to overcome such conflicting interests. Extensive spawning migrations are performed by a variety of species from catfish in the Amazon River (e.g. Jones, 2002) to cod in the Barents Sea (Hjort, 1914). However, finding a spawning location which maximises fitness is a complex decision including favourable retention of offspring (Sinclair and Iles, 1989), temperature (Otterlei et al., 1999), timing (Hjort, 1914; Cushing, 1986), turbulence (Lasker, 1981; MacKenzie and Kiørboe, 2000), predator (Bailey and Houde, 1989) and prey abundance (Cushing, 1990). Biophysical models have been used to simulate the linkage of these variables based on distribution of the spawning stock and oceanographic flow fields (Heath and Gallego, 1998; Hinrichsen et al., 2002; Brickman et al., 2007). Continuous advancements in computer technology has now made it possible to generate high resolution flow fields, and to model not only large scale ocean currents, but also to incorporate smaller features such as local eddies and turbulence. These mesoscale processes, often generating favourable current schemes around spawning or nursery areas, have long been thought to be of great importance in early larval stages (Hjort, 1914). Based on the pioneering larval-recruitment work of Hjort (1914), Sinclair and Iles (1989) introduced what later became known as the member-vagrant hypothesis, advocating the importance of retention of larvae in favourable areas during the early stages. Retention has been known to be important for recruitment for a long time (Bigelow, 1926), and more recent studies have found retention to be crucial for fish recruitment in both tropical and boreal waters (e.g. Sammarco and Andrews, 1988; Lough et al., 2006). Here, we investigate interactions between ocean circulation patterns, retention and temperature, and how these vary with latitude in Northeast Arctic (NEA) cod.
The NEA cod perform extensive southbound upstream migrations from feeding grounds in the Barents Sea to various spawning banks outside the Norwegian coast. In recent decades its spawning migrations have been reduced to a fraction of its historical expansion (Jørgensen et al., 2008). Prior to the 1990s these banks where located on a latitudinal range from the Finnmark Coast (~71º N) to the Møre Coast (~63º N), with the highest spawning density around the Lofoten area at about 69º N. During the last 30 years, cod have abandoned their southernmost spawning banks outside the Møre coast, and concentrate almost solely around the Lofoten area, and also spawning further north, at the Finnmark Coast (Anon., 1910–1976; Mehl, 2004; Sundby and Nakken, MS 2005). Modern fisheries in the Barents Sea have truncated the size distribution, reduced the fraction of older and larger individuals (Ottersen, 2008), and favoured increased energy allocation to reproduction (Sætersdal and Hylen, 1964; Jørgensen, 1990; Jørgensen et al., 2007). There is a strongly held belief that these changes alter the NEA cod’s spawning migrations (Godø, 2003).
After spawning, eggs and larvae undergo a 2–3 month northbound downstream drift back into the Barents Sea where they eventually settle to the bottom as 5–6 months old juveniles. While these simple population-level processes have been known for nearly a century (Hjort, 1914), it is still unknown what might motivate spawning migrations up to twice the distance of their conspecifics, and exposing their offspring to an equivalently long return journey. Potential advantages to offspring could be associated with food availability, predation pressure and/or temperatures. Jørgensen et al. (2008) developed an optimality model suggesting that the fitness benefit to parents (females) from migration is state dependent, since larger or fatter females (better condition) will benefit more from spawning further south relative to smaller and more lean females.
Temperature is important to growth of larval cod (Otterlei et al., 1999; Folkvord, 2005), and several studies have suggested an increased recruitment of NEA cod during warm compared to cold years (Ottersen et al., 2006). We explore the hypothesis that eggs and larvae spawned at southern locations will experience higher integrated ambient temperatures during their early drift phase, and subsequently also have a potential for faster growth. If this is the case, individuals investing in longer southbound migrations could gain a potential fitness benefit by providing more favourable conditions for their offspring. Using an oceanographic model, ROMS, together with a particle tracking model we trace the downstream drift of particles released at six important spawning grounds along the Norwegian coast. The model simulation is run for the years 1985 and 1986 due to their differences in abundance, distribution and growth of the 0-group cod (Ådlandsvik and Sundby, 1994). The total abundance of 0-group cod was higher in 1985 than 1986, the distribution covered a larger area, and the centre of biomass was farther to the west. The average length and weight (Ellertsen et al., 1989), was also significantly higher in 1985 than in 1986 (Ottersen and Loeng, 2000).
The Models
Our main focus in this study has been to single out variation in temperature exposure of eggs or larvae (particles) drifting from different spawning locations along the coast. By using a general ocean circulation model (Haidvogel et al., 2007) and a Lagrangian particle-tracking model (Ådlandsvik and Sundby, 1994) we released batches of individual particles above various spawning grounds and tracked their drift trajectories at fixed depths over a few months. Each individual was assigned with initial horizontal coordinates and depth. A forward integration in time according to modelled ocean circulation provided temperature exposure, from which temperature-dependent growth potential for larval cod could be estimated. Because the particles are suspended at fixed depths and are subjected to passive drift throughout the simulation period, it is irrelevant whether we address them as eggs or larvae with regards to drift and temperature exposure.
We know that vertical and horizontal swimming behaviour is likely to influence the dispersal of particles released in this region (Vikebø et al., 2005, 2007). However, there is limited information about behaviour of larvae in the field, and we assume particles drift passively with the prevailing ocean currents at fixed depths. The reason for this is twofold. Firstly, the particle trace will then return the drift trajectories and temperature exposure at each discrete depth, rather than from a mixture of several depths depending on vertical behaviour. Secondly, we avoid errors introduced through the implementation of vertical behaviour. Although keeping larvae in fixed depths is incorrect, it appears more parsimonious for our purpose here. For the same reasons we do not discriminate between the banks in terms of prey and predator abundance.
Similar modelling studies have had their main focus on the spread and distribution of released particles (Ådlandsvik and Sundby, 1994; Vikebø et al., 2005), and/or the ability of virtual larvae to influence growth and survival by encompassing various behavioural traits (Vikebø et al., 2007). These studies have solely concentrated on particles released from a few places around Lofoten, and the consecutive northbound drift. In this study we explore the explicit effect different spawning grounds and latitudes have on the particle drift, as well as temperature exposure and successive larval growth potential.
Materials and Methods
Ocean Model
The circulation model used in this study is the Regional Ocean Modelling System (ROMS), version 2.0 (Haidvogel et al., 2007). This is a free-surface, hydrostatic, primitive equation ocean model that uses stretched terrain-following coordinates in the vertical and orthogonal curvilinear coordinates in the horizontal.
Monthly mean climatological values of velocity, temperature, salinity, and water elevation in addition to four dominant tidal constituents (M2, S2, K1, and N2) are used to specify the initial conditions and the lateral boundary conditions (Engedahl et al., 1998). Hence, no interannual variation is imposed at the lateral boundaries. The model forcing also includes daily NCEP/NCAR reanalysed wind-stress, air pressure, and ocean-atmosphere heat exchange for the years 1985 and 1986 (Kalnay et al., 1996). The shortwave radiation is multiplied by a factor, which decreases linearly from 1.0 at the southernmost boundary to 0.5 at the northernmost boundary, in order to reproduce measured temperature distributions. The rationale behind this is that the NCEP/NCAR cloud cover for the Barents Sea seems to be too low (Budgell, 2005). Additional forcing is given by prescribed river run-off from 12 freshwater sources along the coast. The vertical model grid consists of 25 sigma-layers. The horizontal resolution increases from about 3.8 km in the southernmost parts, 5.3 km in the Vestfjord, and up to 8.5 km in the northernmost parts of the model domain. The bottom topography is taken from Etopo2, which gives a horizontal resolution of about 3.5 km. A general evaluation of the model shows that it reproduces the observed hydrography and current metre measurements at stations and sections (Vikebø, 2005).
Particle Release and Tracking
The particles are released from six known spawning grounds along the Norwegian coast (Rollefsen, 1960) covering a latitudinal range of more than 650 km (Fig. 1). The chosen grounds are, from south to north, Vikna, Vega, Røst, Vestfjorden, Moskenesgrunnen and Malangsgrunnen. Particles are moved forward by the daily mean updated velocity fields from the “Lagrangian advection and diffusion” hydrodynamic model (Ladim: Ådlandsvik and Sundby, 1994). Earlier work included a random component in addition to advection to parameterize Fickian diffusion (Csanady, 1973; Ådlandsvik and Sundby, 1994), but this is not included in this study, reducing the spread of particles. With decreasing grid size, the need for diffusion is reduced, as the range of resolved eddies and velocity shear is increased, leading to greater spreading of the particles also in deterministic realisations of the model.
Particles are released from 2 March, and tracked for 100 days. During the first 60 days of the simulation period, batches of 25 particles are released every third day. They are released simultaneously at all spawning grounds and at four different depths (5, 10, 20 and 30 m) to cover the upper water column where eggs and larvae are observed. A total of 12 000 particles are traced for each year. This ensures a representative estimate of dispersal from each spawning ground, although climatological forcing is highly variable through the spawning period. We also get to trace each particle for a minimum of 100 days after release.
Temperature-dependent Growth
For each particle we record standard length, growth, and spatial coordinates. We ignore mortality, and individual specific growth rate (SGR, d-1) is an empirical function of body mass and ambient temperature for larval cod fed ad libitum, reared under laboratory conditions (Folkvord, 2005):
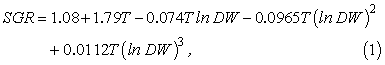
where T is temperature (°C) and DW is body mass in grams dry weight. All individuals are initialized with a dry weight of 0.03 mg, and a length of 3.53 mm (Otterlei et al., 1999). This is used to illustrate how variable growth can be from various spawning locations, and it is not an attempt to simulate any kind of existing prey fields. We acknowledge that temperature-dependence in egg-stage duration has not been included explicitly; therefore our estimation of temperature-effects at early age may be slightly biased. However, growth rates during egg stages are also closely coupled to sea temperatures, and any relative deviations from the larval growth function should not influence our overall results to any significant degree.
Results
We present examples of typical particle drift routes from the spawning grounds of Northeast Arctic cod towards the Barents Sea. Furthermore we show how dispersion, and thus drift trajectories are significantly influenced by seasonality, and also how this affects the retention at the spawning banks. Finally we explore how integrated temperature exposure is dependent on all these factors, and how this governs growth potential
Drift Trajectories and Driving Forces
Through the simulation period we released in total 12 000 particles over six banks and four depths, all comprising individual drift trajectories. Rather than plotting all these trajectories in one figure, we present an example of typical drift routes of particles released on 11 March 1985 (Fig. 2). The figure shows drift trajectories of 25 particles released at 10 m depth from each of the six spawning grounds. Although trajectories change through the season and from day to day, it is clear that particles from all spawning grounds follow the prevailing North-Atlantic current, or the Norwegian coastal current north towards the Barents Sea. There are differences in retention at and around the spawning grounds, and Vikna and Røst appear to have notably stronger retention than the rest. This is quantified as the distance each particle has drifted from its origin during the first four days (Fig. 3). Here we notice a clear relationship between time of release and particle retention above their respective banks. For both 1985 and 1986 particles released early in the season experience rapid advection away from the bank compared to later in the season.
Temperature Exposure and Related Growth
Each individual drift trajectory has a unique temperature signature. The mean temperature exposure for particles released at 10 meters from all banks have been plotted as a function of time since release (particle age) both for 1985 and 1986 (Fig. 4). The standard deviation has been plotted for the spawning grounds with highest and lowest temperature (Vestfjorden and Vikna, respectively) (Fig. 4). It is apparent that particles released at Vikna experience the highest temperatures. Particles released at Vestfjorden experience low temperatures at early age, though the spread in temperatures between all grounds, excluding Vikna, is moderate, especially in 1986.
The effects of latitude and release dates on the particles’ mean temperature exposure are presented in Fig. 5. The highest temperatures are observed at the southern spawning ground, Vikna (64.9º N), apart from 5 m depth in 1986, which seems to be colder across the entire latitudinal range. Particles released later in the season also experience higher temperatures.
To translate the temperature-exposure into relative difference in larval growth-potential over latitude, we have standardized larval weight, averaged over all particles released at a given time and location at the age of 100 days. This was done simply by dividing the size of larvae from each spawning ground with the maximum average value at each date of release. This has been plotted as a function of latitude and release date (Fig. 6). We observe that the southern-most spawning ground (Vikna at 64.9º N) generally has the highest daily growth throughout the simulation period. However, in 1986 at 5 m depth there are tendencies of relatively high growth across the entire latitudinal range.
Discussion
It is evident that this study has brought to light some of the many complexities that even a simple question can raise. Apparently, different spawning grounds encompass properties important to fitness in NEA cod offspring. We observe that temperature exposure of drifting particles may be affected by local retention, which in turn is dependent on local tides, topography, frontal structures and weather conditions. Furthermore, important spawning grounds such as Vestfjorden did not provide the best temperature exposure. Possibly, these areas have a richer supply of food, or other benefits that the model does not include, or, it may be due to a trade-off between adult costs of migrating further and the risk of offspring dispersing too far north if spawning takes place further north (Jørgensen et al., 2008). The strong seasonal variation in drift trajectories, and corresponding temperature exposure, suggest close links to wind driven processes.
Several modelling studies have tracked particles (larvae) from spawning grounds along the Norwegian coast to the Barents Sea (e.g. Ådlandsvik and Sundby, 1994; Vikebø et al., 2007) These studies have mainly focused on one or two spawning grounds with large-scale drift processes, some with vertically and/or horizontally mobile larvae. Here we have focused on how ambient temperature for offspring differ for alternative spawning grounds at a wide latitudinal range. Our simulation predicted that a significant proportion of all particles released, at all depths and spawning grounds, were advected north by the prevailing currents, eventually ending up in the Barents Sea. This prediction is consistent with earlier studies, i.e., a significant proportion of eggs spawned at southern spawning grounds end up in the Barents Sea recruiting to the NEA cod population. (Godø, 1984; Robichaud and Rose, 2004). However, some particles, especially those released near the coast, might never reach the Barents Sea, but end up in various fjord systems. Vikebø et al. (2007) show in their simulation of particle drift from the Lofoten and Moskenes banks that particles distributed deeper in the water column experienced a more easterly distribution in the Barents Sea than those inhabiting shallower water, which drifted further north. They also found that particles released closer to the core of the North Atlantic current are more likely to end up in the northern and western parts of the Barents Sea. In our study we see in fact particles at the same depth divide into the two main drift trajectories when passively following prevailing currents (Fig. 2). However, we observe large variation in the dispersion and distribution of the particles depending on season, and on a day to day basis. Vikebø (2005) found that absolute wind stress, indicating input of kinetic energy to the circulation model, is significantly decreasing between March and May for the year 1985, and on average between 1980 and 1990. In the field, larvae exposed to strong winds may sink deeper to avoid strong turbulence (Visser et al., 2001), reducing the effect of wind on their drift trajectories. Fiksen et al. (2007) showed in a similar model setup that larvae swimming 1–3 body-length s-1 might determine whether they end up in the eastern or northern parts of the Barents Sea by constant, directional swimming.
Seasonality and wind stress do not only influence the terminal destination of the drift trajectories, but also play a crucial role in retention at spawning banks. Retention of larvae in warm favourable conditions is known to be important for recruitment to the stock (Sinclair and Iles, 1989). Hinrichsen et al. (2001) also found that wind driven circulation is a central component in determining the dispersion, and thus recruitment, of the Baltic cod larvae. In our simulations particles released early in the season were generally prone to little retention and consecutive fast northbound drift (Fig. 3). We hypothesize that wind stress is an important factor, perhaps not in generating the retaining circulation itself, but for advecting larvae out of favourable current schemes and potentially exposing them to lower temperatures and reduced growth potential (Figs. 5 and 6).
Conversely, retention does not provide an unconditional recipe for recruitment success. Vestfjorden, known to be an important spawning area for NEA cod, reveals high retention and very low sea temperatures for both 1985 and 1986 in our model and reflect suboptimal temperature conditions for larval survival. However, our model temperatures are consistent with actual observations (Vikebø, 2005). The general circulation in Vestfjorden is cyclonic, and would normally enhance advection out of the fjord (Mitchelson-Jacob and Sundby, 2001). However, frequent low pressure fields early in the season may temporarily mediate these circulation patterns and enhance retention. The spawning grounds in Vestfjorden are known to be of great importance to NEA cod, supporting spawning for a significant proportion of the stock. Thus we must assume that factors such as low predation or high prey abundance (Furnes and Sundby, 1981) may compensate for the suboptimally low sea temperatures.
Disentangling the effects that season, wind stress, retention and drift trajectories have on larval temperature exposure is difficult considering that the variables themselves are inter-dependent. However, the spawning grounds stand out as perhaps the single most important independent variable for determining temperature exposure and growth. Other external drivers might mediate or enhance the effects of the spawning ground, but do not to any profound extent alter their significance to the temperature exposure (Fig. 5). It is clear that larvae released above the southernmost spawning ground, Vikna, experience the highest temperatures and subsequent growth not only as a result of a southern location, but also because of favourable retention schemes – especially in the late season. This positive effect of retention on growth potential at Vikna is somewhat corroborated by the relatively lower temperature at Vega, only 0.7 degrees further north and accompanying slower growth. This suggests that a mere north-south gradient in temperature exposure and thereby growth potential is highly inaccurate, suggesting that a spawning ground’s latitudinal position is not always closely connected to offspring success. However, at a larger scale, considering the historical spawning migration to western and southern parts of Norway, we think the upstream spawning migration by NEA cod is partly associated with temperature benefits to offspring. To further resolve this question, we need to include flow-fields all the way to the southern tip of Norway, and preferably, for a longer time period than two years.
References
ÅDLANDSVIK, B., and S. SUNDBY. 1994. Modelling the transport of cod larvae from the Lofoten area. ICES Mar. Sci. Symp., 198: 379–392.
ANON. 1910–1976. Norges fiskerier (Grandes pêches maritimes). In: Norges offisielle statistikk, Vols 7–12. The Norwegian annual fisheries statistics [In Norwegian]. Department of fisheries, H. Aschehoug & Co., Oslo, Norway.
BAILEY, K. M., and E. D. HOUDE. 1989. Predation on eggs and larvae of marine fishes and the recruitment problem. Adv. Mar. Biol., 25: 1–83.
BIGELOW, H. B. 1926. Plankton of the offshore waters of the Gulf of Maine. Bull. Bur. Fish., 40: 1–509.
BRICKMAN, D., G. MARTEINSDOTTIR, K. LOGEMANN, and I. H. HARMS. 2007. Drift probabilities for Icelandic cod larvae. ICES J. Mar. Sci., 64: 49–59.
BUDGELL, W. P. 2005. Numerical simulation of ice-ocean variability in the Barents Sea region. Towards dynamical downscaling. Ocean Dynamics, 55: 370–387. doi:10.1007/s10236-005-0008-3
CSANADY, G. T. 1973. Turbulent diffusion in the environment. D. Riedel, Rotterdam, the Netherlands. 248 p.
CUSHING, D. H. 1986. The migration of larval and juvenile fish from spawning ground to nursery ground. ICES J. Mar. Sci., 43: 43–49. doi:10.1093/icesjms/43.1.43
1990. Plankton production and year-class strength in fish populations - an update of the match mismatch hypothesis. Adv. Mar. Biol., 26: 249–293.
ELLERTSEN, B., P. FOSSUM, P. SOLEMDAL, and S. SUNDBY. 1989. Relations between temperature and survival of eggs and first feeding larvae of the North-East Arctic cod (Gadus morhua L.). Rapp. P.-V. Reun. Cons. Int. Explor. Mer., 191: 209–219.
ENGEDAHL, H., B. ÅDLANDSVIK, and E. A. MARTINSEN. 1998. Production of monthly mean climatological archives for the Nordic Seas. J. Mar. Syst., 14: 1–26. doi:10.1016/S0924-7963(98)00004-9
FIKSEN, Ø., C. JØRGENSEN, T. KRISTIANSEN, F. VIKEBØ, and G. HUSE. 2007. Linking behavioural ecology and oceanography: larval behaviour determines growth, mortality and dispersal. Mar. Ecol. Prog. Ser., 347: 195–205. doi:10.3354/meps06978
FOLKVORD, A. 2005. Comparison of size-at-age of larval Atlantic cod (Gadus morhua) from different populations based on size- and temperature-dependent growth models. Can. J. Fish. Aquat. Sci., 62: 1037–1052. doi:10.1139/f05-008
FURNES, G. K., and S. SUNDBY. 1981. Upwelling and wind induced circulation in Vestfjorden. In: The Norwegian Coastal Current. R. Saetre and M. Mork (eds.), Vol. 1, p. 152–177.
GODØ, O. R. 1984. Cod (Gadus morhua L.) off Møre - composition and migration. In: The propagation of cod Gadus morhua L. - Flødevigen rapportserier. E. Dahl, D. S. Danielsen, E. Moksness and P. Solemdal (eds.). Institute of Marine Research, Arendal, Norway, p. 591–608.
2003. Fluctuation in stock properties of north-east Arctic cod related to long-term environmental changes. Fish Fish., 4: 121–137. doi:10.1046/j.1467-2979.2003.00112.x
HAIDVOGEL, D. B., H. ARANGO, W. P. BUDGELL, B. D. CORNUELLE, E. CURCHITSER, E. D. LORENZO, K. FENNEL, W. R. GEYER, A. J. HERMANN, L. LANEROLLE, J. LEVIN, J. C. MCWILLIAMS, A. J. MILLER, A. M. MOORE, T. M. POWELL, A. F. SHCHEPETKIN, C. R. SHERWOOD, R. P. SIGNELL, J. C. WARNER, and J. WILKIN. 2007. Ocean forecasting in terrain-following coordinates: Formulation and skill assessment of the Regional Ocean Modeling System. Journal of Computational Physics, 227: 3595–3624. doi:10.1016/j.jcp.2007.06.016
HEATH, M. R., and A. GALLEGO. 1998. Bio physical modelling of the early life stages of haddock, Melanogrammus aeglefinus, in the North Sea. Fish. Oceanogr., 7: 110–125. doi:10.1046/j.1365-2419.1998.00061.x
HINRICHSEN, H. H., M. S. JOHN, E. ARO, P. GRONKJAER, and R. VOSS. 2001. Testing the larval drift hypothesis in the Baltic Sea: retention versus dispersion caused by wind-driven circulation. ICES J. Mar. Sci., 58: 973–984. doi:10.1006/jmsc.2001.1090
HINRICHSEN, H. H., C. MOLLMANN, R. VOSS, F. W. KOSTER, and G. KORNILOVS. 2002. Biophysical modeling of larval Baltic cod (Gadus morhua) growth and survival. Can. J. Fish. Aquat. Sci., 59: 1858–1873. doi:10.1139/f02-149
HJORT, J. 1914. Fluctuations in the great fisheries of northern Europe viewed in the light of biological research. Rapp. P.-V. Reun. Cons. Int. Explor. Mer., 20: 1–228.
JONES, C. M. 2002. Age and growth. In: Fishery science: the unique contributions of early life stages. L. A. Fuiman and R. G. Werner (eds.). Blackwell Science Ltd., Malden, USA, p. 33–63.
JØRGENSEN, C., E. S. DUNLOP, A. F. OPDAL, and Ø. FIKSEN. 2008. The evolution of spawning migrations: the role of individual state, population structure, and fishing-induced changes. (In press).
JØRGENSEN, C., K. ENBERG, E. S. DUNLOP, R. ARLINGHAUS, D. S. BOUKAL, K. BRANDER, B. ERNANDE, A. GARDMARK, F. JOHNSTON, S. MATSUMURA, H. PARDOE, K. RAAB, A. SILVA, A. VAINIKKA, U. DIECKMANN, M. HEINO, and A. D. RIJNSDORP. 2007. Ecology: Managing Evolving Fish Stocks.Science, 318: 1247–1248. doi:10.1126/science.1148089
JØRGENSEN, T. 1990. Long-term changes in age at sexual maturity of Northeast Arctic cod (Gadus morhua L). ICES J. Mar. Sci., 46: 235–248. doi:10.1093/icesjms/46.3.235
KALNAY, E., M. KANAMITSU, R. KISTLER, W. COLLINS, D. DEAVEN, L. GANDIN, M. IREDELL, S. SAHA, G. WHITE, J. WOOLLEN, Y. ZHU, M. CHELLIAH, W. EBISUZAKI, W. HIGGINS, J. JANOWIAK, K. C. MO, C. ROPELEWSKI, J. WANG, A. LEETMAA, R. REYNOLDS, R. JENNE, and D. JOSEPH. 1996. The NCEP/NCAR 40-year reanalysis project. Bull. Am. Meteorol. Soc., 77: 437–471. doi:10.1175/1520-0477(1996)077<0437:TNYRP>2.0.CO;2
LASKER, R. 1981. The role of stable ocean in larval fish survival and subsequent recruitment. In: Marine fish larvae. Morphology, ecology, and relation to fisheries. R. Lasker (ed), University of Washington Press, Seattle, USA, p. 80–87.
LOUGH, R. G., E. A. BROUGHTON, L. J. BUCKLEY, L. S. INCZE, K. P. EDWARDS, R. CONVERSE, A. ARETXABALETA, and F. E. WERNER. 2006. Modeling growth of Atlantic cod larvae on the southern flank of Georges Bank in the tidal-front circulation during May 1999. Deep-Sea Res. (II Top. Stud. Oceanogr.),53: 2771–2788.
MACKENZIE, B. R., and T. KIØRBOE. 2000. Larval fish feeding and turbulence: A case for the downside. Limnol. Oceanogr., 45: 1–10.
MEHL, S. MS 2004. Abundance of spawning Northeast Arctic cod spring 2004. Internal Cruise Report, Institute of Marine Research, Bergen, Norway, ISSN 1503-6294, No. 9, p. 26.
MITCHELSON-JACOB, G., and S. SUNDBY. 2001. Eddies of Vestfjorden, Norway. Cont. Shelf Res., 21: 1901–1918. doi:10.1016/S0278-4343(01)00030-9
OTTERLEI, E., G. NYHAMMER, A. FOLKVORD, and S. O. STEFANSSON. 1999. Temperature- and size-dependent growth of larval and early juvenile Atlantic cod (Gadus morhua): a comparative study of Norwegian coastal cod and Northeast Arctic cod. Can. J. Fish. Aquat. Sci., 56: 2099–2111. doi:10.1139/cjfas-56-11-2099
OTTERSEN, G. 2008. Pronounced long-term juvenation in the spawning stock of Arcto-Norwegian cod (Gadus morhua) and possible consequences for recruitment. Can. J. Fish. Aquat. Sci., 65: 523–534. doi:10.1139/F07-185
OTTERSEN, G., D. O. HJERMANN, and N. C. STENSETH. 2006. Changes in spawning stock structure strengthen the link between climate and recruitment in a heavily fished cod (Gadus morhua) stock. Fish. Oceanogr., 15: 230–243. doi:10.1111/j.1365-2419.2006.00404.x
OTTERSEN, G., and H. LOENG. 2000. Covariability in early growth and year-class strength of Barents Sea cod, haddock, and herring: the environmental link. ICES J. Mar. Sci., 57: 339–348. doi:10.1006/jmsc.1999.0529
ROBICHAUD, D., and G. A. ROSE. 2004. Migratory behaviour and range in Atlantic cod: inference from a century of tagging. Fish Fish., 5: 185–214. doi:10.1111/j.1467-2679.2004.00141.x
ROLLEFSEN, G. 1960. Norwegian fisheries research; Translations of articles from "Havet og våre fisker". Grieg, Bergen, Norway, p. 1–53.
SÆTERSDAL, G., and A. HYLEN. 1964. The decline of the skrei fisheries. Reports on Norwegian Fishery and Marine Investigations, 13: 58–69.
SAMMARCO, P. W., and J. C. ANDREWS. 1988. Localized dispersal and recruitment in Great Barrier-Reef corals - the Helix experiment. Science, 239: 1422–1424. doi:10.1126/science.239.4846.1422
SINCLAIR, M., and T. D. ILES. 1989. Population regulation and speciation in the oceans. ICES J. Mar. Sci., 45: 165–175. doi:10.1093/icesjms/45.2.165
SUNDBY, S., and O. NAKKEN. MS 2005. Spatial shifts in spawning habitats of Arcto-Norwegian cod induced by climate change. GLOBEC International Newspaper, 11: 26.
VIKEBØ, F. B. 2005. The impact of climate on early stages of Arcto-Norwegian cod - a model approach, Geophysical Institute. University of Bergen, Norway, 141 p.
VIKEBØ, F. B., C. JØRGENSEN, T. KRISTIANSEN, and Ø. FIKSEN. 2007. Drift, growth and survival of larval Northeast Arctic cod with simple rules of behaviour. Mar. Ecol. Prog. Ser., 347: 207–219. doi:10.3354/meps06979
VIKEBØ, F. B., S. SUNDBY, B. ÅDLANDSVIK, and Ø. FIKSEN. 2005. The combined effect of transport and temperature on distribution and growth of larvae and pelagic juveniles of Arcto-Norwegian cod. ICES J. Mar. Sci., 62: 1375–1386. doi:10.1016/j.icesjms.2005.05.017
VISSER, A. W., H. SAITO, E. SAIZ, and T. KIORBOE. 2001. Observations of copepod feeding and vertical distribution under natural turbulent conditions in the North Sea. Marine Biology, 138: 1011–1019. doi:10.1007/s002270000520
WILLIAMS, G. C. 1966. Natural selection costs of reproduction and a refinement of Lacks principle. American Naturalist, 100: 687–690. doi:10.1086/282461
|