Introduction
Fish spatial distribution has been shown to be non-random, relating to density-dependent or -independent processes (Anderson and Gregory, 2000; Fromentin et al., 2001; Julliard et al., 2001). Although density-dependent processes, such as inter- and intra-specific competition and predation, are important in structuring species distributions (Shepherd and Litvak, 2004), impacts of abiotic environmental variables cannot be ignored.
The abiotic environmental variables can include a wide range of factors. Temperature is often considered to be one of the most important environmental variables determining the spatial range of fish species over many ecosystems (Munday et al., 2008; Selleslagh and Amara, 2008). For example, some temperature-sensitive species, like spiny dogfish (Squalus acanthias) and silver hake (Merluccius bilinearis), may show distinct changes in distribution based on bottom temperature variability (Methratta and Link, 2007a); while for some other species, like Atlantic cod (Gadus morhua), juvenile and adult move according to changes in the ambient water temperature and the movements were related to length and maturity levels (Jones and Campana, 2009). Many studies have found that depth is another important environmental driver of fish spatial distribution; and the effects of depth are more obvious in more open areas than in riverine and coastal marine ecosystems (Magnussen, 2002; Jaureguizar et al., 2006; Methratta and Link, 2007a; Sanchez et al., 2008). Salinity, with numerous discharges of freshwater along the coast, is also one of the key factors in influencing the spatial distribution of fish in many ecosystems (Lazarri and Stone, 2006; Childs et al., 2008). The salinity changes have been shown to affect primary productivity levels in the Gulf of Maine (Ji et al., 2008), potentially having further affects up the food chain. Besides that, spatial locations defined by longitude and latitude are also important (Tolimieri and Levin, 2006); and in coastal regions, distance offshore may also affect fish distribution (Jaureguizar et al., 2007).
Our understanding of the habitat and the role it plays in regulating fish community structures have become ever more important with the enactment of the Sustainable Fisheries Act of 1996 in the United States, which included a legislative mandate for the identification and the protection and enhancement of essential fish habitat (16 U.S.C. Sec 1801). Congress’ recognition of the importance of habitat to the survival, and at this stage, the rebuilding of fish stocks, enforces the need to understand stock distributions and their relations to the surrounding environment.
A good understanding of the spatial distribution of fish species, especially commercially-valuable species, is central to successful fisheries management. During the last two decades, the Gulf of Maine fish community has experienced a substantial change. The groundfish stocks have suffered a large decrease in landings since the early 1990s, from 19 036 metric tonnes (mt) in 1991 to most recent 3 554 mt in 2007 (Maine DMR, 2008). The lobster industry on the other hand has increased greatly over the same time period with landings doubling and even tripling compared with historical catches (Maine DMR, 2008). Similar trends can also been found in the fishery independent data (ASMFC, 2000; NEFSC, 2005). These species have shown some consistent environmental associations over the U.S. fishing history (Wahle and Steneck, 1992; Methratta and Link, 2006, 2007a, b).
The traditional single-species management, along with the inconsistencies between the scales of ecological processes and the management system and the lack of understanding of ecosystem structure and functions, might have contributed, at least partly, to the current depleted status (USCOP, 2004). The push both federally and at the local level for an ecosystem-based management approach, along with satisfying and fully implementing the requirements of the Sustainable Fisheries Act, requires a finer analysis of species’ distributions and habitat associations.
Although the community structure has a long history in ecological research, there were limited publications on the quantitative analysis of the fish community and their habitats in the Gulf of Maine area. Dijkstra et al. (2007) has compared the temporal pattern of ascidians species and some studies have analyzed the fish community of the Gulf of Maine but focused on the biodiversity (Witman, 1996; Jordaan, 2006) or stability (Bertness et al., 2002) of the system. With such evidence for the role of environmental factors in driving distributions, a need exists to quantify these associations.
In this paper, we evaluated seasonal and annual variations in the spatial distribution of the fish community in the coastal Gulf of Maine using data collected from a fishery-independent inshore bottom trawl survey. Key environmental drivers have been shown to drive spatial patterns in the fish community structure. We predicted that these relationships would be quantifiable within our dataset, further demonstrating the importance of the abiotic factors in structuring fish distributions. The results derived from this study provide the information critical in improving our understanding of the spatial and temporal variation in the fish community structure, which is essential to developing ecosystem-based fisheries management.
Materials and Methods
Study site
The Gulf of Maine (Fig. 1) is a complex and variable shelf water body, covering nearly 103 000 km2 (Townsend, 1997; Balch et al., 2008). Its approximate 12 000 km shoreline (Stauble, 2004) comprises various inlets, bays, estuaries, and coastal communities, extending into the Atlantic Ocean nearly 320 km. It averages 150 m in depth (O’Brien, 1999) with a maximum depth of 275 m (Uchupi and Austin, 1987). Fresher Labrador Slope water entering the Gulf via the Northeast Channel between Browns Bank and Georges Bank (Ramp et al., 1985), along with nearshore flow coming along the southern tip of Nova Scotia (Smith, 1983), contribute to the Gulf’s complexity and signature water column. The bottom topography off the northern coasts of the Gulf is dominated by narrow ridges, small pinnacles, and numerous small channels (Uchupi, 1968).
Economically, the inshore Gulf of Maine represents an important source of income and support for many coastal Maine communities. These communities have seen a drop in landings by over 50% in most species, with a sole positive trend in lobster landings (Maine DMR, 2008). The inshore fisheries support 26 000 jobs, directly and indirectly related to the seafood industry, bringing US$860 million per year to the Maine state economy (Schmitt, 2004).
Survey design
The ability to understand habitat interactions with the fish community is directly related to the ability to survey fish species and their habitats, and the environmental characteristics of the water column. The most commonly used survey gear is trawling, such as otter trawls or beam trawls. Sampling with trawls can overcome depth restrictions, covering a consistent transect width and time, while being cost-effective. However, fisheries surveys in general are susceptible to the differing catchability coefficients for target species (Auster et al., 2001; Sanchez et al., 2008), possibly leading to biased estimates of fish compositions within the targeted fish community. This, however, should not be a problem for a study focused on the spatial and temporal variations in fish community, as long as survey catchability for a given fish species is relatively constant spatially and temporally. Further, if such catchability is spatially and temporally constant, the fish composition from a trawl survey could be considered as a proxy for the larger fish community structure (Auster et al., 2001).
Based upon these assumptions, we used the data from the "Maine-New Hampshire Inshore Bottom Trawl Survey" conducted by the Maine Department of Marine Resource (DMR), which began in the autumn of 2000 and continued every spring and autumn since that time. The data series we used terminates in 2007. The stratified random design of the survey divides the inshore Gulf of Maine off the coast of New Hampshire and Maine into four depth strata: 9–37 m; 37–64 m; 64–100 m; and 100+ m (Fig. 1), along with five longitudinal regions along the coast. Due to the area’s peculiar geography, these zones are divided along longitude rather than latitude, as one moves northeast from the New Hampshire-Massachusetts border to the international line between Maine and Canada. The boundaries of these regions are based on oceanographic, geological, and biological features along the diverse coast (Fig. 1). Each sampling season has a target site number target set at 100 sites, which amounts to a density of one station per 137 km2 of the targeted survey area (Sherman et al., 2005).
The sampling gear is a modified shrimp net not designed to target any particular species but rather to target many near-bottom, dwelling species. The net has 2-inch mesh in the wings and ½-inch mesh liner in the cod end. The foot rope and head ropes are 57-feet and 70-feet, respectively, with 6-inch rubber cookies. The gear was designed to be light on the bottom to minimize habitat disruptions. Every tow lasted approximately twenty minutes, depending on bottom type and lobster trap interferences. To avoid bias with different tow lengths the survey abundance were standardized by the tow distance (Sherman et al., 2005).
Environmental variables
We chose to focus on six environmental variables. In addition to depth, salinity, and bottom temperature, we used two spatial variables including the survey’s longitudinal regions. To add an additional spatial component, being aware of the depth and hydrodynamic changes that can occur with increasing movement onto the shelf, we also used distance from shore as an environmental driver. These environmental variables are chosen for their potential direct or indirect effect on fish distribution.
When the survey abundance was recorded, the five environmental characteristics were also recorded simultaneously for each sampling station, including longitudinal strata (numbers-LONSTA), distance to the shore (meters-DIS), depth (fathoms-DEP, 1 fathom = 1.8288 m), bottom temperature (ºC-TEMP) and bottom salinity (Salinity-SAL). The DIS is transformed from longitude (Lon) and latitude (Lat) by using the equation:
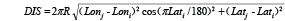
where R is the earth radius, i is the survey location, j is the closest point to the survey station in the shoreline.
Besides above environmental variables, we also include substrate type (numbers-SUBTYP). There are four main habitat types: (1) gravelly, (2) sandy, (3) rocky, and (4) muddy/clay. The composite substrate type, sand with subordinate gravel, was used as the fifth substrate type in our study, because it covers most of the all composite substrate stations. The other composite substrate type, such as rock with subordinate gravel and gravel with subordinate rock, which only cover a small percentage of the whole survey stations (~2%), we use the dominant textures of that station as the substrate types.
The main geological map we used to characterize the seafloor materials is from Barnhardt et al. (1998) with the scale 1:100 000. However, that map only covers the Maine coastal area and omits the coastal areas sampled off the New Hampshire coast. For the New Hampshire stations, we used the substrate map from Poppe et al. (2005) with the resolution of 0.00001 decimal degrees.
Statistical analyses
A large number of fish species was selected in our study based on two criteria: (1) the individual of that species was surveyed at least in one station every year, and (2) the average annual appearance frequency was larger than 30%. Those species were also divided into subgroups using hierarchical cluster analysis (CA). The maximum likelihood estimation and Bayesian Information Criterion (BIC) were applied to identify the most likely number of clusters; and R Mclust package for R v.2.11.1 was used to implement CA.
To visualize the data, we applied principal component analysis (PCA) to both the fish abundance data and the environmental data to quantify their spatial structures. PCA is an ordination multivariate technique and has been commonly used for reducing the dimensions of multivariate data without losing inherent information offering an effective way to summarize data of a multivariate nature (McGarigal et al., 2002, p. 19–23). Additionally PCA allows assessment of the larger physical processes, which result from interactions of the individual environmental components like salinity and temperature, in combination with spatial variables of distance from shore and along-shore positioning. Although some other multivariate statistical analyses can also be used for similar analysis, PCA has been one of the most commonly used methods in fisheries ecological studies (Chen et al., 2008). All fish abundance data were log-transformed and standardized prior to the data processing.
Regression models were developed for quantifying the relationship between the spatial structure of fish species and the environmental variables. Given that the data from the grouped fishes had been well abstracted in the PCA, we used the first principal component (PC) of each species group instead of the original fish data and set them as the dependent variables. A better fit of the regression model indicates that the fish abundance would be highly influenced by the environmental variables. Such a PCA-based regression analysis approach can overcome the problem of possible correlations between the environmental variables, which are used as the independent variables in the regression analyses (Chen et al., 2008).
Results
Spatial variability of fish community and environmental variables
A total of 155 species were recorded in the Maine-New Hampshire Inshore Bottom Trawl Survey. We narrowed our study to 29 species for autumn surveys and 25 species for the spring surveys (Fig. 2), which represented 75.4% and 73.6% of the total surveys records, respectively. These species include not only commercial fishes and invertebrate species but also other species, which may have important roles in the Gulf of Maine ecosystem.
By using cluster analysis, the 29 selected autumn survey species were divided into three species-subgroups (Fig. 2a); while the 25 selected spring survey species were divided into two species-subgroups(Fig. 2b).
The temporal patterns of the key fish species distributions could be documented using the survey results. The spiny dogfish shifted their distribution southward during autumn over the eight survey years (Fig. 3). The silver hake shifted their distribution between the inshore and offshore areas over the autumn surveys (Fig. 4). The Atlantic cod stock abundance was low prior to 2004 and appeared to increase in the southern area after the 2004 autumn survey (Fig. 5). Some species were consistently higher in overall abundance compared to other species caught in the Maine-New Hampshire Inshore Bottom Trawl Survey, such as American lobster (Homarus americanus) and Atlantic herring (Clupea harengus) (Fig. 6 and Fig. 7) compared to other species such as goosefish (Lophius americanus) and windowpane flounder (Scophthalmus aquosus). For other species, the annual variations of the spatial distribution observed were relatively low. Compared with the autumn surveys, there were fewer observable trends in the spring surveys.
The deepest depth of the survey was approximately 195 m. The bottom temperature ranged from 2.68ºC to 13.69ºC, and the autumn temperature was generally higher than the spring temperature. The salinity of the near bottom layer varied from 27.8 PSU to 34.4 PSU, and the average salinity in autumn was also higher than that in the spring. Over the annual time series of bottom temperature, the values in 2002 and 2006 were always above the average; while the values in the 2004 were always below the average. The annual time series of salinity had similar patterns, though the trend was not as apparent as that in bottom temperature.
Principal component analysis
In the PCA, the first few PCs often explain most of the variations inherent in the original data. Additionally, the weightings of the principal components help identify what contributed most to the differences between the individual sites. In our study, the proportions of the eigenvalues for the environmental variables in each season indicated that the first two PCs provided a good summary of the information inherent in the data. Together, the first two PCs explained about 74% of the standardized variance in the data, with the first PC accounting for approximately 48% of the standardized variance (Table 1).
Fig. 8 shows the eigenvectors of the first two PCs for the environmental variables in the autumn 2003 survey. The first PC was a combination of all environmental variables except for longitudinal strata. In autumn surveys, temperature shows positive loading (~0.4) on PC1, while depth, salinity, substrate type and distance to the shore, display high negative loadings (~-0.5). However, in spring surveys the loadings of environmental variables have similar values but of opposite signs. The second PC was a combined measure of longitudinal strata and temperature, showing high negative loadings (<-0.5) in autumn surveys but high positive loadings (>0.5) in spring surveys (Fig. 8a, b). Additionally, seasonal variation of the eigenvectors from the first two PCs was evident. These results were consistent throughout the survey years.
We performed a similar PCA on the log-transformed survey abundance data of various species group and subgroups for each survey. The length of the arrow to each species is proportional to the variance of the species and indicates how well the species is represented by the PCs. The plots of the PC scores for the location numbers in the planes of PC1 and PC2 help to locate the survey stations of the fish species. An example using autumn 2003 data showed that the PC1 contrasted sites with high abundance of species subgroup 1, such as rock crab and haddock, due to their positive values (Fig. 9a), and species subgroup 2, such as northern shrimp and witch flounder, due to their negative values (Fig. 9b). The PC2 showed negative loadings for most of the species in subgroup 3, such as American lobster and silver hake (Fig. 9c). In addition, we also identified regional trends, with the stations in the northeast gathered in the bottom section of the plots (Fig. 9). The greater the stations’ scores on the PCs from the vector origin were the higher anomalous localization of the species. For example, the large negative score of Station 18 on PC2 indicated the high catch rate of species subgroup 2 at this survey station (Fig. 9). However, the interpretation of the PCs on the survey abundance data was not as obvious as with the environmental variables. The first two PCs for the abundance data only accounted for 43% of the standardized variance, which likely resulted from a lack of correlation among many fish species.
After grouping the species into our three subgroups, we found that the eigenvalues within each subgroup of species were lower. The first PC accounted for 27%, 53%, and 28% of the standardized variances of species subgroup 1, 2, and 3, respectively; and the first two PCs accounted for 46%, 66%, and 49% of the standardized variances of the species subgroup 1, 2, and 3, respectively (Fig. 10).
Regression analyses
The first two PCs of the environmental variables were both significant as the independent variables in the regression model. Again, we used the autumn 2003 survey data as an example. The regression model of the PC1 of the overall species group versus the first two PCs of the environmental variables was significant (R2 = 0.846, p<0.01). The estimated regression model was PC1grp=1.3(5.7%)×PC1env-1.17(9.6%)×PC2env, where numbers in the parentheses are coefficients of variance. In this model the intercept for all species group and subgroups was not significantly different from zero and therefore excluded. The coefficients of the environmental PCs for all species group and subgroups from each survey were included in Fig. 11.
Most of the regression coefficients for the overall species group and subgroups were clustered together for both the autumn and spring surveys, showing that the linear relationships were coherent among years (Fig. 11). However, annual variation for the relationship between spatial structures of some species subgroups and environmental variables were evident, for example, species subgroups 1 and 3 in autumn surveys and subgroup 2 in spring surveys.
From the summary of the R2-values for the species groups in the regression analyses, we concluded that the R2-values of the overall species group were higher than those of the subgroups (Table 2). Within the species subgroups, the R2-values for the subgroup 2 were higher than the other subgroups in the autumn surveys. In general, the R2-values were higher during the autumn than in the spring surveys (Table 2).
Discussion
This study evaluated inshore trawl survey data to quantify spatial relationships among and between species groups and environmental variables within the coastal Gulf of Maine. A PCA was used to abstract spatial structures of both environmental data and fish survey abundance data allowing for identification of the linear relationships between environmental variables and species composition.
We restricted our study to the most frequently surveyed species in order to better understand their general patterns in tempo-spatial distributions in the ecosystem. This restriction kept our focus on species that had higher levels of occurrences. In doing so we likely ignored some of the smaller scale events, which likely need to be resolved on a finer scale, due to season- and year-specific environmental effects, such as weather and other finer-scale environmental influences.
The grouping of species was essential to identifying spatial associations. Dividing the species into related groups increased the amount of explained variance by the first two PCs, making it possible to use the first two PCs to represent the spatial structure of the fish survey abundance. The grouping was based upon cluster analysis results of appearance data, rather than other morphological or anecdotal-based groupings in order to avoid bias in later statistical analysis results. The relatively higher explained variances for the species subgroup 2 could be attributed to the similar distribution patterns of its component species. These species may have similar behaviors, such as sharing particular habitat for resource or predation reasons as has been shown for flatfish species (McConnaughey and Smith, 2000) or benthic invertebrates (ASMFC, 2000).
The spatial structures of species subgroups, for example subgroup 2 in the autumn surveys, were well-characterized by the environmental variables, as evidenced by the regression models between the PCs (Table 2). However, not all species within these subgroups were necessarily influenced equally by the environmental variables or influenced equally during the different sampling seasons. For example, silver hake are known to be temperature-sensitive (Methratta and Link, 2007a). Thus temperature through PC2 may have had a higher influence on the distribution of this species compared with others subgroups (Brooks and Johnston, 1993). Our grouping, although able to help distinguish certain trends in spatial association, may have led to some lumping of results for fish species.
The environmental PCs included both environmental and spatial variables. PC1 likely reflected inshore-offshore processes addressing depth, salinity, distance to the shore, and substrate type differences. Species responses to changes in depth and salinity have been shown to effect their distributions in terms of nearshore versus more offshore locations (Gunderson et al., 1990; Jones and Campana, 2009). PC2 reflects along-shore movement, with high loading on the longitudinal variable. Additionally though, PC2 showed high loading from temperature, possibly indicating an effect of circulation along the coast (Pettigrew et al., 1998).
The relationships between the spatial structures of most fish subgroups and the environmental variables were not stable. For example, the species subgroup 2, though the R2-values are higher, the regression coefficients varied among surveys (Fig. 11). Steneck (1997) highlighted the changes in the structure of the fish community along the Maine coast, including that the community was in a temperate, alternative equilibrium, which may not be temporally stable over large time scales. Our results support such a conclusion, as we could find little coherence in the regression parameters for species groups between seasons and among years. Additionally, environmental changes co-occurring with species shifts could alter the basic relationships among the species.
The annual difference in regression coefficients could have also been due to missing of additional environmental variables. Circulation studies showed that in 2000, for example, a continuous flow along the coast was observed, whereas 2001 represented a year with partial flow-through down the coast and partial veering of the Eastern Gulf of Maine Coastal Currents into the deeper Gulf of Maine (Pettigrew et al., 2005). Witman (1996) indicated water turbidity might influence the dispersal and settlement of larvae of some species. Also, the concentrations of nutrient and dissolved oxygen may enhance or reduce the primary and thus the secondary production (Townsend and Pettigrew, 1997). In this study we addressed the major environmental variables that showed consistent forcing across many studies, but additional variables along with biotic variables may need to be incorporated to capture more precisely the spatial structure of the fish community (Jaureguizar et al., 2006).
Although catchability among all of the species surveyed was not equal, as the survey covered complex-seeking benthic invertebrates, pelagic species, and sand-burrowing flatfish, the maintenance of catchability of these different species across spatial and temporal changes, using the standard protocol for the survey, does lend itself to assessing the spatial structures of fish species (Auster et al., 2001).
Our use of PCs to summarize the environmental data and fish community data, over other methods, is important to note. Jackson (1993) discussed stopping rules in PCA, which involve the evaluation of statistical significance of PCs leading to problems of "effective degrees of freedom" (North et al., 1982). This issue is important when we need to use a large number of PCs in further analyses because it is likely some of the PCs may not be statistically significant. This study, however, only uses the first PC for the fish community data and the first and second PCs for the environmental variables, as they are likely to be statistically significant (Jackson, 1993). However, if we were to decide to include additional PCs, their significance would need to be thoroughly evaluated.
Aside from the PCA, there are other ordination methods that are conceptually similar and could have been applied in this study, such as the detrended correspondence analysis (Aguilar-Perera and Appeldoorn, 2008; Matthews et al., 1992), the canonical correspondence analysis (Jackson and Harvey, 1993) and non-metric multi-dimensional scaling (Diggins and Newman, 2009). We chose the PCA over the other approaches because, (1) it is an effective and most commonly used ordination multivariate technique to discover the structure of high-dimensional data when the data are narrowly lying near a linear subspace, and (2) the new variables (PCs) derived from PCA are uncorrelated with each other.
With the increased emphasis on ecosystem-based management, and as required by the legislation (16 USC 1801 1996; Fleeger and Becker, 2008), the need to understand species distributions and interactions with environmental variables becomes more important. The Maine-New Hampshire Inshore Trawl survey provides a sufficient data set to analyze the distributions of fish species in the coastal Gulf of Maine. Although its reliability for some fish subgroups is uncertain, it offers an additional resource for studying the fish community structure along the coastal Gulf of Maine. Our results indicate that environmental variables can be related to species distributions in this area. With future analysis considering more years and associations, predictions may be possible based on these environmental factors. While many of the commercial ground fish stocks appear depleted, the rebuilding process is essential for continued economic and ecological recovery. The ability to rebuild the productivity of important exploited stocks in the Gulf of Maine also depends upon the understanding and functioning of their spatial structure. This study contributes to the process of yielding the required information in the development of ecosystem-based fisheries management in the coastal Gulf of Maine.
Acknowledgements
This research is in part supported by the Maine Sea Grant College program through a NOAA research grant (NA06OAR4170108) to Y. Chen and the University of Maine fellowships to Y. Zhang, D. Brzezinski and J. H. Chang. Bottom trawl surveys were financially supported by the Maine Department of Marine Resources. We would like to thank Sally Sherman for helping provide the survey data. We would like to extend our appreciation to the two anonymous reviewers and the editors Dr. A. Thompson and Dr. H.-J. Rätz for their valuable comments, which greatly improved the manuscript.
References
AGUILAR-PERERA, A., and R. APPELDOORN. 2008. Spatial distribution of marine fishes along a cross-shelf gradient containing a continuum of mangrove-seagrass-coral reefs off southwestern Puerto Rico. Estuar. Coast. Shelf Sci., 76: 378–394. doi:10.1016/j.ecss.2007.07.016
ANDERSON, J., and R. GREGORY. 2000. Factors regulating survival of northern cod (NAFO 2J3KL) during their first 3 years of life. ICES J. Mar. Sci., 57: 349–359. doi:10.1006/jmsc.1999.0530
ASMFC. 2000. American Lobster Stock Assessment Report for Peer Review. Atlantic State Marine Fisheries Commission, Providence, RI. Rep. No. 00–01: 532pp.
AUSTER, P., K. JOY, and P. VALENTINE. 2001. Fish species and community distributions as proxies for seafloor habitat distributions: the Stellwagen Bank National Marine Sanctuary example (Northwest Atlantic, Gulf of Maine). Environ. Biol. Fish., 60: 331–346. doi:10.1023/A:1011022320818
BALCH, W., D. DRAPEAU, B. BOWLER, E. BOOTH, L. WINDECKER, and A. ASHE. 2008. Space-time variability of carbon standing stocks and fixation rates in the Gulf of Maine, along the GNATS transect between Portland, ME, USA, and Yarmouth, Nova Scotia, Canada. J. Plankton Res., 30: 119–139. doi:10.1093/plankt/fbm097
BARNHARDT, W. A., J. T. KELLEY, S. M. DICKSON, and D. F. BELKNAP. 1998. Mapping the Gulf of Maine with side-scan sonar: a new bottom-type classification for complex seafloors. J. Coast. Res., 14: 646–659.
BERTNESS, M., G. TRUSSELL, P. EWANCHUK, and B. SILLIMAN. 2002. Do alternate stable community states exist in the Gulf of Maine rocky intertidal zone? Ecology, 83: 3434–3448. doi:10.1890/0012-9658(2002)083[3434:DASCSE]2.0.CO;2
BROOKS, S., and I. A. JOHNSTON. 1993. Influence of development and rearing temperature on the distribution, ultrastructure and myosin sub-unit composition of myotomal muscle-fibre types in the plaice Pleuronectes platessa. Mar. Biol., 117: 501–513.
CHEN, Y., X. CHEN, and L. XU. 2008. Developing a size indicator for fish populations. Sci. Mar. (Barc.), 72: 221–229.
CHILDS, A., P. COWLEY, T. NAESJE, A. BOOTH, W. POTTS, E. THORSTAD, and F. OKLAND. 2008. Do environmental factors influence the movement of estuarine fish? A case study using acoustic telemetry. Estuar. Coast. Shelf Sci., 78: 227–236. doi:10.1016/j.ecss.2007.12.003
DIGGINS T. P., and A. M. NEWMAN. 2009. Environmental and spatial influences on benthic community composition in wooded headwater streams in Zoar Valley, New York, USA. Hydrobiologia, 630: 313–326. doi:10.1007/s10750-009-9824-7
DIJKSTRA, J., L. G. HARRISA, and E. WESTERMANA. 2007. Distribution and long-term temporal patterns of four invasive colonial ascidians in the Gulf of Maine. J. Exp. Mar. Biol. Ecol., 342: 61–68. doi:10.1016/j.jembe.2006.10.015
FLEEGER, W., and M. BECKER. 2008. Creating and sustaining community capacity for ecosystem-based management: is local government the key? J. Environ. Manage., 88: 1396–1405. doi:10.1016/j.jenvman.2007.07.018
FROMENTIN, J., R. MYERS, O. BJORNSTAD, N. STENSETH, J. GJOSAETER, and H. CHRISTIE. 2001. Effects of density-dependent and stochastic processes on the regulation of cod populations. Ecology, 82: 567–579. doi:10.1890/0012-9658(2001)082[0567:EODDAS]2.0.CO;2
GUNDERSON, D., D. ARMSTRONG, Y. B. SHI, and R. A. MCCONNAUGHEY. 1990. Patterns of estuarine use by juvenile English sole (Parophrys vetulus) and Dungeness crab (Cancer magister). Estuaries, 13: 59–71. doi:10.2307/1351433
JACKSON, D. A. 1993. Stopping rules in principal components analysis: a comparison of heuristical and statistical approaches. Ecology, 74: 2204–2214. doi:10.2307/1939574
JACKSON, D. A., and H. H. HARVEY. 1993. Fish and benthic invertebrates: Community concordance and community-environment relationships. Can. J. Fish. Aquat. Sci., 50: 2641–2651. doi:10.1139/f93-287
JAUREGUIZAR, A., R. MENNI, C. LASTA, and R. GUERRERO. 2006. Fish assemblages of the Northern Argentine Coastal System: spatial patterns and their temporal variations. Fish. Oceanogr., 15: 326–344. doi:10.1111/j.1365-2419.2006.00405.x
JAUREGUIZAR, A., J. WAESSLE, and R. GUERRERO. 2007. Spatio-temporal distribution of Atlantic searobins (Prionotus spp.) in relation to estuarine dynamics (Rio de la Plata, Southwestern Atlantic Coastal System). Estuar. Coast. Shelf Sci., 73: 30–42. doi:10.1016/j.ecss.2006.12.012
JI, R., C.DAVIS, C.CHEN, D. TOWNSEND, D. MOUNTAIN, and R. BEARDSLEY. 2008. Modeling the influence of low-salinity water inflow on winter-spring phytoplankton dynamics in the Nova Scotian Shelf-Gulf of Maine region. J. Plankton Res., 30: 1399–1416. doi:10.1093/plankt/fbn091
JONES, J., and S. CAMPANA. 2009. Stable oxygen isotope reconstruction of ambient temperature during the collapse of a cod (Gadus morhua) fishery. Ecol. Appl., 19: 1500–1514. doi:10.1890/07-2002.1
JORDAAN, A. 2006. Determining environmental drivers of fish community structure along the coast of Maine. PhD dissertation, School of Marine Sciences, University of Maine, Orono, ME 04469, USA.
JULLIARD, R., N. STENSETH, J. GJOSAETER, K. LEKVE, J. FROMENTIN, and D. DANIELSSEN. 2001. Natural mortality and fishing mortality in a coastal cod population: a release-recapture experiment. Ecol. Appl., 11: 540–558.
LAZZARI, M., and B. STONE. 2006. Use of submerged aquatic vegetation as habitat by young-of-the-year epibenthic fishes in shallow Maine nearshore waters. Estuar. Coast. Shelf Sci., 69: 591–606. doi:10.1016/j.ecss.2006.04.025
MAGNUSSEN, E., 2002. Demersal fish assemblages of Faroe Bank: species composition, distribution, biomass spectrum and diversity. Mar. Ecol. Prog. Ser., 238: 211–225. doi:10.3354/meps238211
MAINE DMR. 2008. Historical Maine Fisheries Landings Data. Maine Department of Marine Resources. http://www.maine.gov/dmr/commercialfishing/historicaldata.htm
MATTHEWS, W., D. HOUGH, and H. ROBSION. 1992. Similarities in fish distribution and water quality patterns in streams of Arkansas: congruence of multivariate analyses. Copeia, 1992: 296–305. doi:10.2307/1446191
MCCONNAUGHEY, R. A., and K. R. SMITH. 2000. Associations between flatfish abundance and surficial sediments in the eastern Bering Sea. Can. J. Fish. Aquat. Sci., 57: 2410–2419. doi:10.1139/cjfas-57-12-2410
MCGARIGAL, K., S. CUSHMAN, and S. STAFFORD. 2002. Multivariate Statistics for Wildlife and Ecology Research. Springer, 283 p.
METHRATTA, E., and J. LINK. 2006. Seasonal variation in groundfish habitat associations in the Gulf of Maine-Georges Bank region. Mar. Ecol. Prog. Ser., 326: 245–256. doi:10.3354/meps326245
METHRATTA, E., and J. LINK. 2007a. Ontogenetic variation in habitat association for four groundfish species in the Gulf of Maine-Georges Bank region. Mar. Ecol. Prog. Ser., 338: 169–181. doi:10.3354/meps338169
METHRATTA, E., and J. LINK. 2007b. Ontogenetic variation in habitat associations for four flatfish species in the Gulf of Maine-Georges Bank region. J. Fish Biol., 70: 1669–1688. doi:10.1111/j.1095-8649.2007.01428.x
MUNDAY, P., J. GEOFFREY, M. PRATCHETT, and W. ASHLEY. 2008. Climate change and the future for coral reef fishes. Fish Fish., 9: 261–285. doi:10.1111/j.1467-2979.2008.00281.x
NEFSC. 2005. Assessment of 19 Northeast Groundfish Stocks Through 2004. In: 2005 Groundfish Assessment Review Meeting (2005 GARM), Northeast Fisheries Science Center, Woods Hole, Massachusetts, 15–19 August, 2005. R. K. Mayo and M. Terceiro (eds.). Northeast Fisheries Science Center Reference Document, 05–13.
NORTH, G. R., T. L. BELL, R. F. CAHALAN, and F. J. MOENG. 1982. Sampling errors in the estimation of empirical orthogonal function. Mon. Weather Rev., 110: 699–706. doi:10.1175/1520-0493(1982)110<0699:SEITEO>2.0.CO;2
O'BRIEN, L. 1999. Factors influencing the rate of sexual maturity and the effect on spawning stock for the Georges Bank and Gulf of Maine Atlantic cod Gadus morhua stocks. J. Northw. Atl. Fish. Sci., 25: 179–203. doi:10.2960/J.v25.a17
PETTIGREW, N., J. CHURCHILL, C. JANZEN, L. MANGUM, R. SIGNELL, A. THOMAS, D. TOWNSEND, J. WALLINGA, and H. XUE. 2005. The kinematic and hydrographic structure of the Gulf of Maine Coastal Current. Deep-Sea Res. (II Top. Stud. Oceanogr.), 52: 2369–2391.
PETTIGREW, N., D. TOWNSEND, H. XUE, J. WALLINGA, P. BRICKLEY, and R. HETLAND. 1998. Observations of the Eastern Maine Coastal Current and its offshore extensions in 1994. J. Geophys. Res., 103: 30623–30639. doi:10.1029/98JC01625
POPPE, L. J., S. J. WILLIAMS, and V. F. PASKEVICH. 2005. USGS East-Coast Sediment Analysis. Procedures, Database, and GIS Data: U.S. Geological Survey Open-File Report 2005–1001, DVD. http://woodshole.er.usgs.gov/openfile/of2005-1001/
RAMP, S., J. SCHLITZ, and W. WRIGHT. 1985. The deep flow through the Northeast Channel, Gulf of Maine. J. Phys. Oceanogr., 15: 1790–1808. doi:10.1175/1520-0485(1985)015<1790:TDFTTN>2.0.CO;2
SANCHEZ, F., A. SERRANO, S. PARRA, M. BALLESTEROS, and J. CARTES. 2008. Habitat characteristics as determinant of the structure and spatial distribution of epibenthic and demersal communities of Le Danois Bank (Cantabrian Sea, N. Spain). J. Mar. Syst., 72: 64–86. doi:10.1016/j.jmarsys.2007.04.008
SCHMITT, C. 2004. Coastal and marine issues. University of Maine Cooperative Extension Plan of Work Issue Area needs Assessment, 2007–2011. University of Maine Cooperative Extension, Orono, ME.
SELLESLAGH, J. and R. AMARA. 2008. Environmental factors structuring fish composition and assemblages in a small macrotidal estuary (eastern English Channel). Estuar. Coast. Shelf Sci., 79: 507–517. doi:10.1016/j.ecss.2008.05.006
SHEPHERD, T., and M. LITVAK. 2004. Density-dependent habitat selection and the ideal free distribution in marine fish spatial dynamics: considerations and cautions. Fish Fish., 5: 141–152. doi:10.1111/j.1467-2979.2004.00143.x
SHERMAN, S., K. STEPANEK, and J. SOWLES. 2005. Maine-New Hampshire Inshore Groundfish trawl survey, Procedures and Protocols. Boothbay Harbor, ME, Maine Department of Marine Resources, p. 42. http://www.maine.gov/dmr/library/dmrresearchrefs.htm
SMITH, P. 1983. The mean and seasonal circulation off southwest Nova Scotia. J. Phys. Oceanogr., 13: 1034–1054. doi:10.1175/1520-0485(1983)013<1034:TMASCO>2.0.CO;2
STAUBLE, D. 2004. Development of a national-scale inventory of shoreline change data for identification of erosion and accretion. Vicksburg, US Army Corps of Engineers, Engineer Research and Development Center, Coastal and Hydraulics Laboratory. http://www.nationalshorelinemanagement.us/docs/NationalScaleInventoryWorkingDraft.pdf
STENECK, R. 1997. Fisheries-induced biological changes to the structure and function of the Gulf of Maine Ecosystem, Plenary Paper. In: Proceedings of the Gulf of Maine Ecosystem Dynamics Scientific Symposium and Workshop. G. T. Wallace and E. F. Braasch (eds.). Regional Association for Research on the Gulf of Maine, RARGOM Report 97–1, Hanover, NH, p. 151–165.
TOLIMIERI, N., and P. LEVIN. 2006. Assemblage structure of Eastern Pacific groundfishes on the U.S. continental slope in relation to physical and environmental variables. Trans. Am. Fish. Soc., 135: 317–332. doi:10.1577/T05-092.1
TOWNSEND, D. 1997. Biochemical cycling of carbon and nitrogen in the Gulf of Maine. In: Proceedings of the Gulf of Maine Ecosystem Dynamics. G. Braasch, and G. Wallace (eds.). Scientific Symposium and Workshop, 16–20 Sept. 1996, St. Andrews, N.B. Regional Association for Research on the Gulf of Maine, RARGOM Report, Hanover, NH, p. 117–134.
TOWNSEND, D., and N. PETTIGREW. 1997. Nitrogen limitation of secondary production on Georges Bank. J. Plankton Res., 19: 221–235. doi:10.1093/plankt/19.2.221
UCHUPI, E. 1968. Atlantic continental shelf and slope of the United States – physiography. US Geological Survey, Professional Paper, 529–C, 30 p.
UCHUPI, E., and J. AUSTIN, 1987. Morphology. Georges Bank. R. H. Backus (ed.). Massachusetts Institute of Technology press, Boston, p. 25–39.
USCOP. 2004. An Ocean Blueprint for the 21st Century, Final Report. United States Commission on Ocean Policy, Washington, DC. http://www.oceancommission.gov/documents/full_color_rpt/000_ocean_full_report.pdf
WAHLE, R., and B. STENECK. 1992. Habitat restrictions in early benthic life: experiments on habitat selection and in situ predation with the American lobster. J. Exp. Mar.Biol. Ecol., 157: 91–114. doi:10.1016/0022-0981(92)90077-N
WITMAN, J. D. 1996. Dynamics of Gulf of Maine benthic communities. In: Workshop on the Health of the Gulf of Maine Ecosystem: Cumulative Impacts and Multiple Stressors. D. Dow and E. Braasch (eds.). Hanover, NH, USA, p. 51–69.
|