J. Northw. Atl. Fish. Sci., Vol. 56: 1–10
Darrell R.J. Mullowney1* and Mackenzie J.E. Mullowney2
1Fisheries and Oceans Canada, Northwest Atlantic Fisheries Centre, 80 East White Hills Road,
St. John’s, NL, Canada, A1C 5X1
2St. Paul’s Junior High, 340 Newfoundland Drive, St. John’s, NL, Canada, A1A 3R9
*darrell.mullowney@dfo-mpo.gc.ca
Mullowney, D.R.J., and Mullowney, M.J.E. 2025. Dynamics of indigenous rock crab (Cancer irroratus) and invasive Green Crab (Carcinus maenas) populations during invasion of a southern Newfoundland estuary. J. Northw. Atl. Fish. Sci., 56. 1–10. https://doi.org/10.2960/J.v56.m754
Abstract
Little literature exists on green crab (Carcinus maenas) and particularly rock crab (Cancer irroratus) in Newfoundland (Canada) waters. In this study, we document demographic composition for the two species upon invasion of green crab into the North Harbour, St. Mary’s Bay, estuary, an area previously only occupied by rock crab and undergoing progressive warming. Using data collected via a citizens science approach, we address objectives of documenting the rate of change in speciation upon green crab invasion in the estuary and providing novel data on basic life history processes for rock crab and green crab in Newfoundland waters. The study shows a rapid proportional switch in species composition in the estuary upon green crab invasion, with green crab increasing from a proportion of 0 to 0.75 of collected samples within three years of being detected. Novel data on rock crab suggest overall consistencies with life history processes as described in the broader literature, including presence of molting periods centred near May and August, a shallow water mating migration in fall, and a similar size-at-instar structure as crab along the eastern United States. Novel data on green crab biology suggests the St. Mary’s Bay population has similar life history attributes as the original invading population in Newfoundland, including spring-summer spawning and an inferred paucity of molting in fall. Allometric carapace relationships of the North Harbour green crab are the same as those described in the Pacific and northeast Atlantic oceans. The size structure of green crab in the North Harbour estuary has broadened in the three years since initial detection with increased presence of large crab beyond the supposed size-at-maturity in recent years.
Keywords: Citizens Science, Green Crab, Invasive Species, Newfoundland, Rock Crab
PDF
Download Citation Data

Citation to clipboard
 Reference management software (Endnote, Mendeley, RefWords, Zotero & most other reference management software)
Reference management software (Endnote, Mendeley, RefWords, Zotero & most other reference management software)
LaTex, BibDesk & other specific software
Introduction
Rock crab (Cancer irroratus) and green crab (Carcinus maenas) are similar species that occupy similar habitats. Both species have large ranges. Newfoundland represents the northern limit for both species in the Northwest Atlantic, with rock crab spanning as far south as Florida (Squires, 1990) and green crab as far south as Virginia (Kingsley, 1879). Within these expansive ranges, both species show considerable plasticity in phenotypic traits to enhance survival and reproduction across a broad spectrum of localized environmental conditions. As ocean warming has progressed in recent decades, both species have invaded new northern territories. Green crab expanded into Newfoundland waters circa 2007 (Best, 2017) and rock crab were first reported in Icelandic waters in 2006 (Gíslason et al., 2021). As invasions have ensued, interactions between the two species have become more commonplace.
In Newfoundland, rock crab is an indigenous species that is found in shallow near-to-shore waters off all coasts. However, despite its commonality and supporting a fishery averaging 80 t per year from 2007 to 2019 (DFO, 2025a), the species has received very little historic research focus and little is known about its basic biology. There is no known information on growth or mortality rates or interactions within the Newfoundland marine ecosystem (DFO, 2025a). Generally, from our observations, we purport they are found on gravelly or rocky shoreline areas (i.e. 0–20 m depth) of bays and estuaries, often in conjunction with kelp or eelgrass beds.
Green crab is an invasive species in Newfoundland, initially found in Placentia Bay on Newfoundland’s south coast, with further expansions in distribution since confirmed along the south and west coasts (DFO, 2025b). Expansion within Newfoundland waters includes into portions of St. Mary’s Bay (Lehnert et al., 2018). This particularly adaptive species is a widespread invader of all continents except Antarctica (Young and Elliott, 2020). Green crab can inhabit a wide range of habitats but highest abundances of juveniles can often be found in sheltered shoreline areas where the intertidal zone consists of rocky substrate and sea grass beds (Klassen and Locke, 2007). Green crab are also adaptive to a wide range of temperature and salinity conditions. The population originally discovered in Placentia Bay is thought to be a cold tolerant hybridized variant with lineages from populations in Nova Scotia and Iceland/Norway (Blakeslee et al., 2010; Jeffrey et al., 2017).
Our study site is the North Harbour estuary in St. Mary’s Bay, Newfoundland (Fig. 1). The estuary is formed where the North Harbour river empties into St. Mary’s Bay. The estuary widens as it extends seaward, but just south of our study beach a spit of land intrudes into the estuary and creates a calm, sheltered embayment. The shoreline consists of small rock and cobble, and there are eelgrass beds located just below the low water mark. From our observations, the estuary itself is overall shallow (i.e. < 10 m depth) and features muddy bottom in the mid portions of it. Salmonids, scallops, and rock crab are among the most common species found in the area.
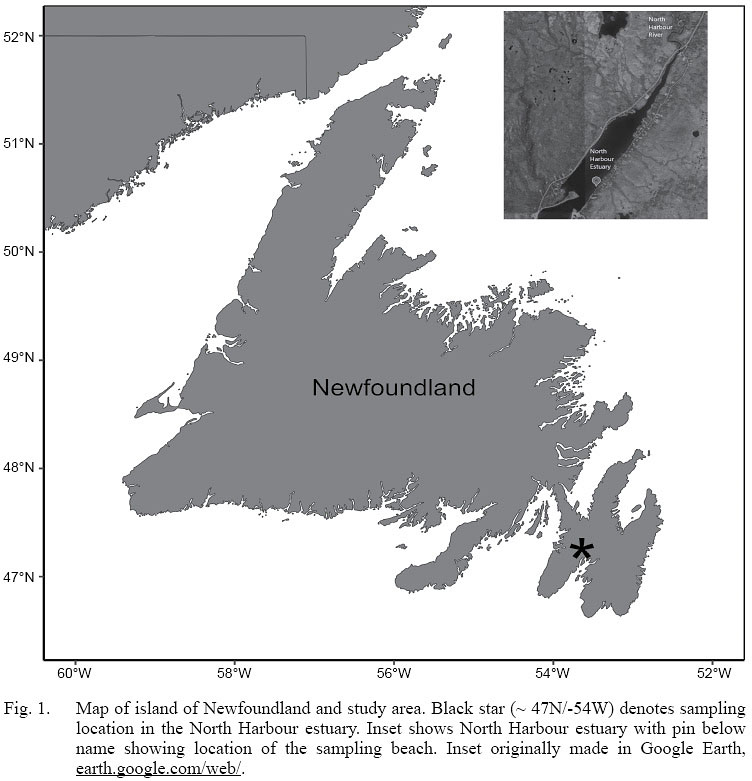
Fig. 1
In this study, we utilize a six-year time series of data consisting of measurements of crab shells taken from opportunistic collections from the North Harbour beach. During this period we documented the first occurrence of invasive green crab into the estuary. Our objectives are to document the rate of change in speciation of the crab community in the North Harbour estuary upon invasion by green crab, and to provide inferences on basic life history traits of rock crab and green crab in the area. These observations constitute some of the first published biological information on rock crab in Newfoundland.
Methods
We began opportunistically collecting and measuring rock crab shells washed up along an approximately 500 m stretch of beach in North Harbour, St. Mary’s Bay, Newfoundland (Fig. 1), in 2019. We measured the shells, both cast dorsal carapaces and whole animals, to one-eighth of an inch (3.2 mm) for carapace width (CW) and carapace length (CL) using a standard tape measure, with both measurements made to the tips of spines. We subjectively assigned shell deterioration to a scale of 1 to 3, by units of 0.5, as a proxy for time on the beach. Bleached (white colouration, dry appearance) shells were scored a 1 and freshest (dark colouration, wet appearance) shells were scored a 3.
We continued collecting and measuring rock crab shells in the same fashion on opportunistic trips to the beach over the 2020–2024 period (Table 1), standardizing the area covered by limiting collections to a portion of the beach confined by landmarks at both ends. In 2021, the first green crab shell was found on the beach, thus we expanded our collections and measurements to green crab, with all measurements taken in the same way as for the rock crab.
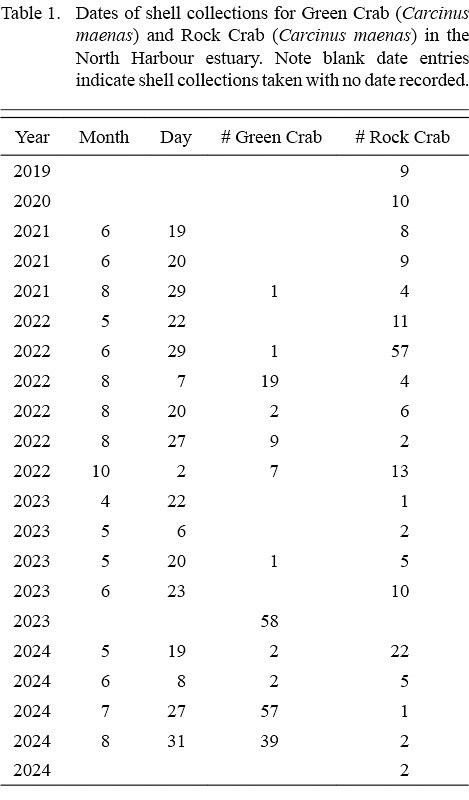
Table 1
Our collections were intended to be a thorough census of the shells on the beach during any given sampling event, but we deem it likely that shells were missed in all or some collection events. Accordingly, and further considering that the time period between collections was variable, we focused population demographic analyses on proportion rather than absolute abundances. Population demographic assessments consisted of examinations of annual proportion of shells collected by species as well as annual width-frequency distributions by species. Mean CW and standard deviations were plotted for each species by sampling event to investigate structural change over time. CW and CL were converted to millimetres for all analyses.
To examine allometric growth relationships between CW and CL morphometrics, we produced scatter plots of the measurements by year. Initial analysis fitting the annual distributions with linear regressions showed little difference in either species, thus the data were pooled for fitting linear regressions to them to estimate allometric relationships.
As a proxy to estimate molt timing in any given year we calculated the proportion of fresh shells (scored as 2 or higher in our visual subjective index) for individual sampling events for both species, with a high proportion interpreted as being indicative of recent molting.
To qualitatively interpret the effect that temperature could have in affecting rate of speciation change, molt timing, or growth rate differences, we downloaded sea surface data from the European Union Copernicus Marine Service (CMS) ocean climate database (i.e. “GLORYS”), accessing the Operational Mercator Global Ocean Reanalysis (OMGOR, 2025; https://doi.org/10.48670/moi-00021) monthly data at 1/12 of a degree confined to a spatial area bound by -53.8 to -53.4W and 47.0 to 47.2N. Monthly means across stations spanning a period from November 2017 to November 2024 were plotted and fit with a linear regression to assess trends in water temperature adjacent to the beach during the study period.
Results
The annual proportion of rock crab shells observed in sample collections shifted from 1 to about 0.25 from 2020 to 2023 as relative green crab prevalence inversely increased (Fig. 2).
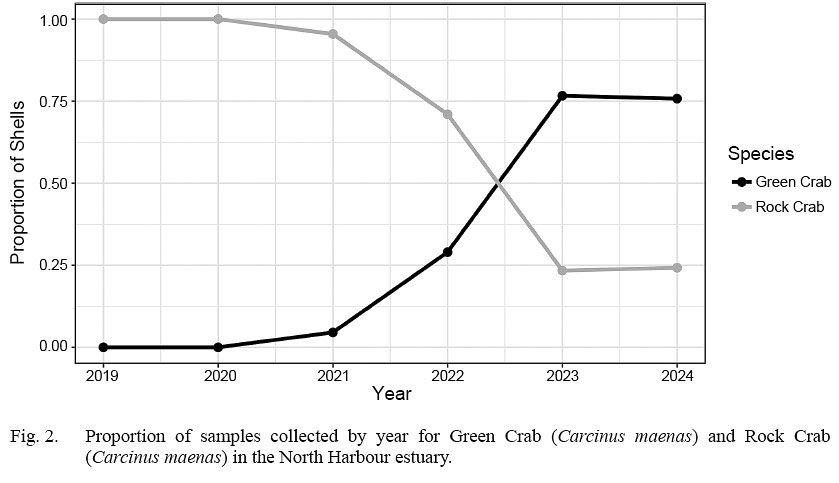
Fig. 2
The majority of rock crab collected ranged from 45 to 90 mm CW (Fig. 2). There was high variability in modal patterns both across and within years, although dominant peaks appeared at about 60–62 mm CW, and 75–76 mm CW, with lower peaks at about 70–71 mm CW and 80 mm CW. The dominant 60–62 mm CW concentration reflected catches in 2021 and 2023, with the secondary 70–71 mm concentration bolstered by 2019 and 2024 samples and the 80 mm concentration notable in 2020 and 2022 catches (Fig. 3).
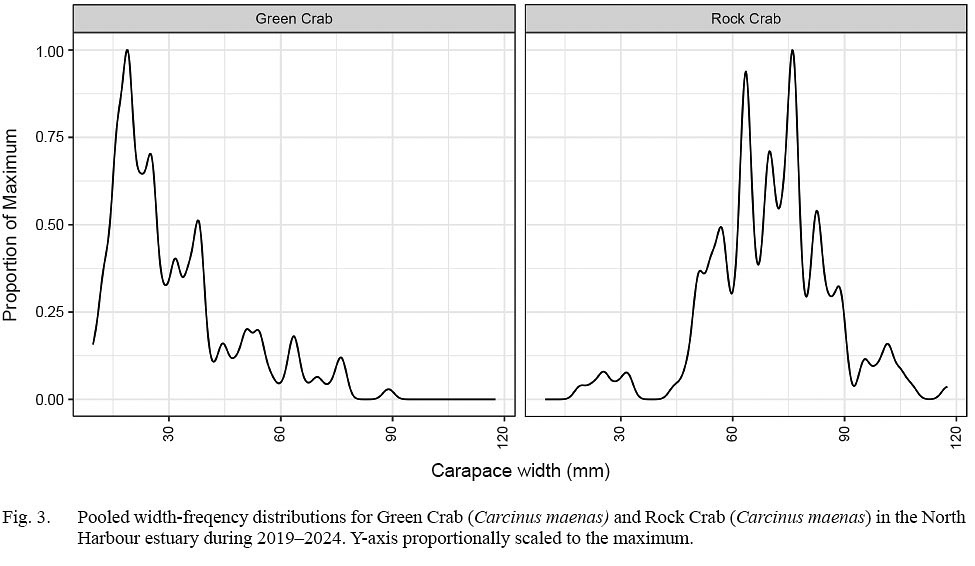
Fig. 3
The majority of green crab collected were below 45 mm CW (Fig. 3), with a primary concentration ranging about 20–25 mm CW present in all three years of collections. The population structure appeared to broaden in 2024, with a secondary concentration centred near 38 mm CW and increasing prominence in concentrations of larger crab near 52 and 75 mm CW (Fig. 4).
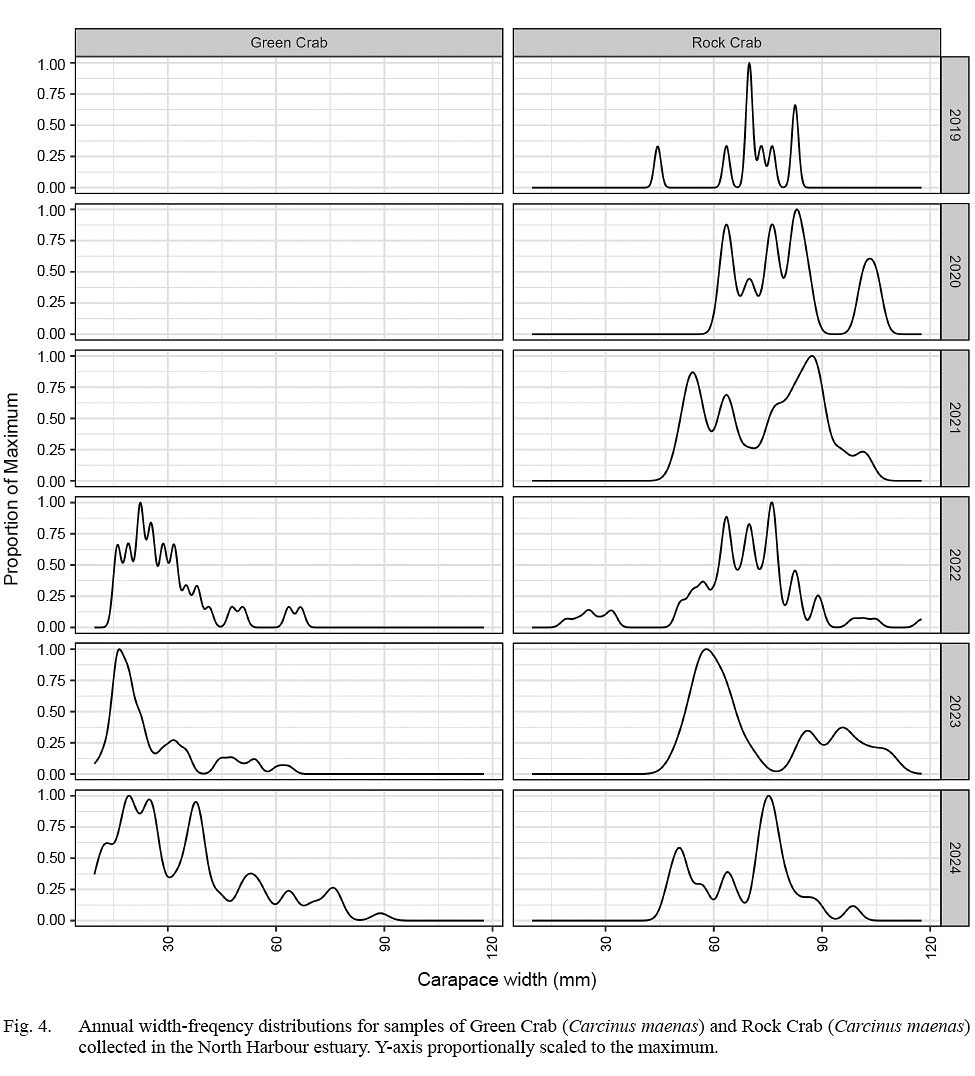
Fig. 4
Mean sizes of rock crab in individual sampling events varied without trend over the study period while mean sizes of green crab systematically increased, doubling from 22.1 mm CW on Aug. 7, 2022 to 44.5 mm CW on June 8, 2024 (Fig. 5).
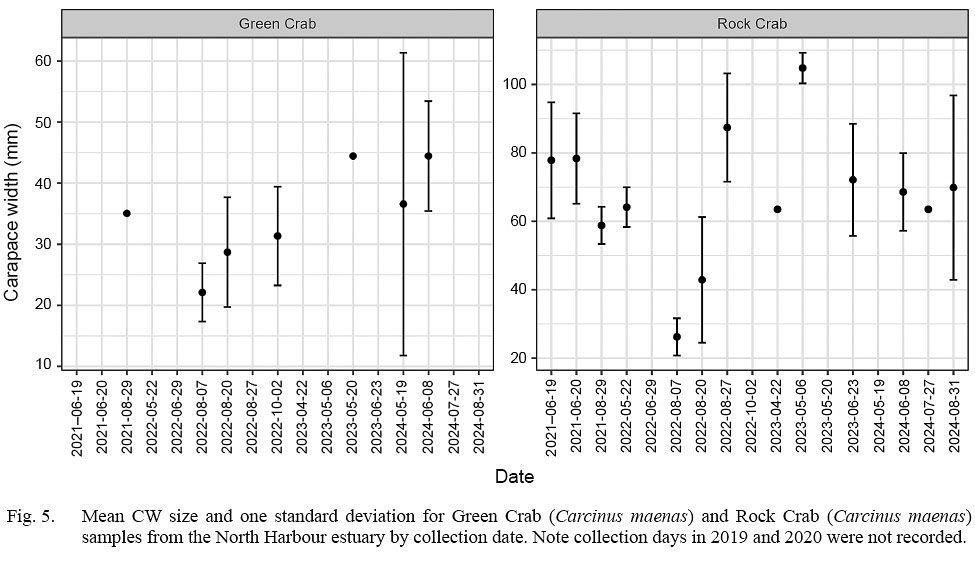
Fig. 5
There were no clear differences in CW-CL relationships across year for either species (Fig. 6). For both species, linear regressions fit to pooled data fit well, with a R2 of 0.93 for green crab and a R2 of 0.89 for rock crab. For green crab, CWs were on average 29% larger than CLs (slope of 1.29) while for rock crab CWs were on average 37% larger than CLs (slope of 1.37).
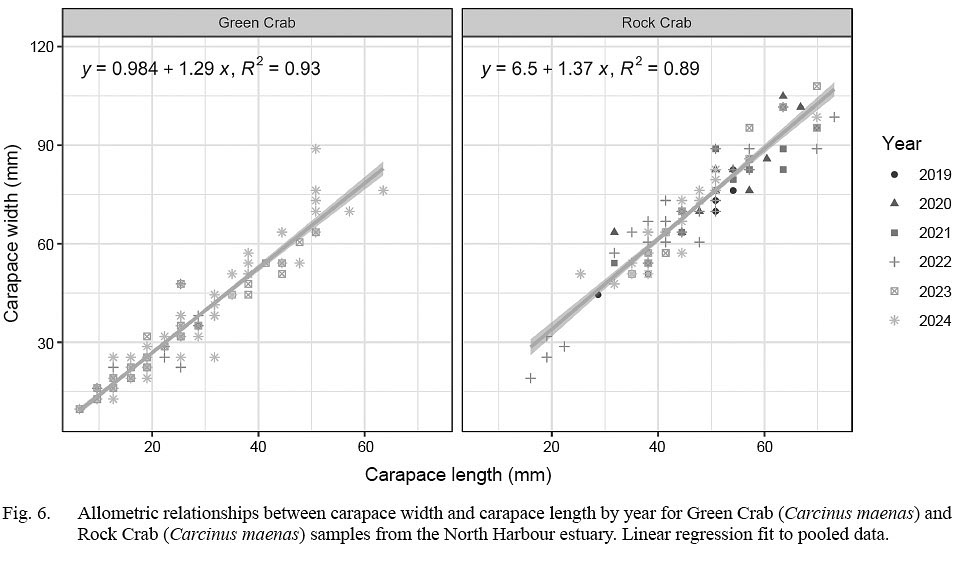
Fig. 6
The proportion of fresh shells in the rock crab samples were at 1 in August in 2021 and 2022 and May in 2023 and 2024, suggesting molting periods at about these months (Fig. 7). The proportion of fresh shells in the green crab samples was near one in most collection periods, suggesting frequent molting. However, there were exceptions to high proportions of fresh shells in green crab samples during collections in August 2022 and July and August in 2024. This suggests a paucity of molting in mid- to late summer in the green crab.
Sea surface temperatures consistently peaked in August and were lowest in March preceding and throughout the study period (Fig. 8). The temperature trend in the linear regression gradually increased at a rate of 0.00033 degrees per month (slope=0.000331) over the study period. The initial Nov. 2017 estimate of 9.75°C was 0.25°C below the terminal Nov. 2024 estimate of 10°C.
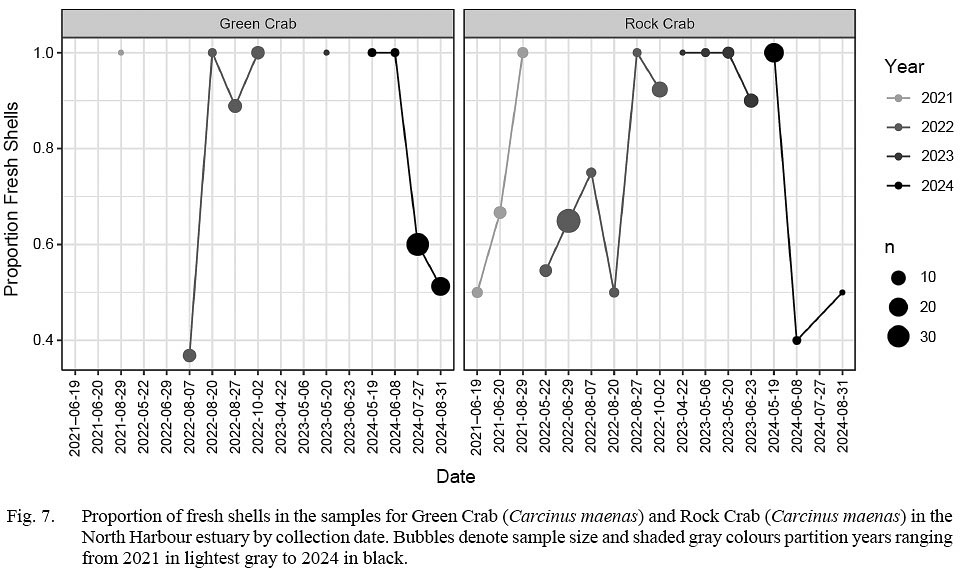
Fig. 7
Discussion
Species interactions
Invasive green crab have substantially altered composition of the crab community in the North Harbour estuary in recent years, with a proportional switch in measured species composition from 0 to 0.75 within three years (2021–2023) of being detected. However, given the non-systematic nature of our collection design and inability to examine absolute abundances of crab, we cannot determine the extent to which this switch in ratio represents an increase in abundance of green crab versus a decrease in rock crab abundance.
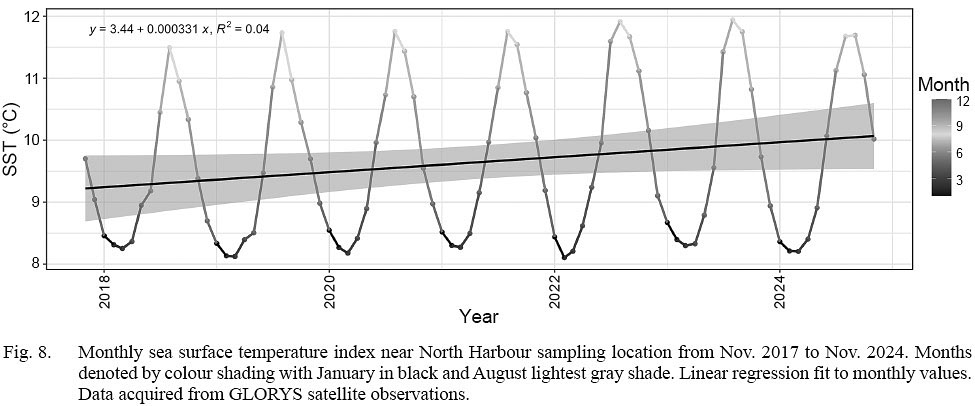
Fig. 8
In terms of interspecific interactions, green crab are generally considered the more aggressive of the two species, as testament by their widespread success as an alien invader (Klassen and Locke, 2007). However, there is literature to suggest that both green and rock crab can be superior competitors upon invasions. For example, in laboratory observations on crab from Newfoundland, Matheson and Gagnon (2012) suggested that green crab can reduce foraging success of small rock crab, particularly in warm water. However, conversely, upon rock crab invasion into Icelandic waters, Gíslason et al. (2021) documented how rock crab were able to become more abundant than the indigenous green crab on soft substrates in years following invasion. In a combined laboratory and field experiment in Prince Edward Island, Belair and Miron (2009) showed that interspecific competition did not affect predation rates or stomach contents for either species, and suggested they tend to avoid each other and can coexist in the wild. More recently, Rossong (2016) noted moderate overlap in habitat and prey preference between the two species in Placentia Bay. In terms of rock crab resilience to green crab presence in the North Harbour estuary, it is important to note that as in Placentia Bay there may be partial habitat partitioning between the two species, and that distributional shifts toward deeper water are a known response of rock crab to green crab invasions (Therriault et al., 2008; Rossong, 2016). Species biology for rock crab is such that they tend to occupy deeper water outside of their reproductive periods (Rebach, 1987; Gendron, 2001), thus a more restricted confinement to deeper habitats could be an outcome for rock crab upon the increased presence of green crab near the beach. Rossong (2016) highlighted that the density of green crab was ultimately the factor that would regulate the extent of food availability and shelter loss for rock crab.
Interactions of green crab with rock crab in shoreline areas are likely to be highest during fall months when rock crab are seeking shallow water for reproduction (Rebach, 1987). However, in terms of predation mortality, it would not be intuitive to expect a substantial direct mortality on rock crab under current population structure in the North Harbour estuary. For example, to-date there has been a notable low proportion of large animals observed in the burgeoning green crab population, with the average size of observed rock crab much larger than green crab.
Despite more time and further observations necessary to assess the extent to which the indigenous rock crab population has been or will be affected by invasive green crab, the expectation is that green crab and associated elevated interspecific competition are in the North Harbour estuary to stay. The physical habitat appears very suitable. For example, the estuarine low flow conditions featuring rock cobble shoreline and dense patches of near-to-shore eelgrass are all positive features for green crab habitat. Moreover, the warming conditions bolster the suitability of habitat for the species.
At this point, one major unknown on the future success of green crab revolves around reproduction dynamics. It is noteworthy that none of our collections were knowingly sexually mature animals, thus the extent to which recruitment is produced from localized spawning versus exogenous factors is unknown. Nonetheless, the progressively broadening size structure of animals in the green crab population would align with increasing potential for localized spawning and increasingly firm establishment of the population.
Rock crab population demographics
Rock crab in Newfoundland are at the northern limit of their North American range distribution, and despite being distributed around the entire island, very little information on their basic biology in Newfoundland waters exists. Our results offer insights into their basic biology, and ultimately show overall consistencies with what is known in the broader literature from other areas. For example, there are two known molting periods in rock crab, one for males well before the fall to ensure their shells are hardened before mating, and one for females prior to reproduction in fall to ensure a soft-shell condition during mating (Page, 2002). Our inference of molting periods centred near May and August align with both processes. Moreover, the absence of crab below about 45 mm CW in our samples suggests we may have been tracking a deep-shallow water mating migration. To elaborate, sexual maturity in other Atlantic provinces can occur at sizes of 55–60 mm CW for females and 70 mm CW for males (Squires, 1990), and mature crab are known to move into shallow water in fall for mating (Rebach, 1987). Accordingly, our late summer molting period inference coupled with a size distribution indicative of dominance by sexually mature crab suggests this migration toward the shoreline for mating was being tracked in our shell collections. As the crab can grow to about 40 mm CW within two years (Squires, 1990),it is apparent that our collections poorly captured frequently molting young crab, thus we infer they are distributed further from the beach in deeper habitats.
With respect to growth, our patterns of concentration in rock crab width-frequency distributions reflected interannual variability, but there were dominant concentrations at about 60–62 mm CW and 75–76 mm CW and secondary concentrations at 70–71 mm CW and 80 mm CW in the data. Little is known about size-at-instar in rock crab, but Bigford (1979) estimated modal CW sizes of 66 and 80 mm for instar X and IX males and modal CW sizes of 61, 71, and 80 mm CW for instars X, XI, and XII in females from New England. They showed primary modes near 60 and 85 mm CW in males and 55 and 85 mm CW in females ranging from Cape Cod to Cape Hatteras and 75 mm CW in males and 65 mm CW in females from North Carolina. Notwithstanding variability in our samples, as well as spatiotemporal differentiation in growth rates, the inference is that our dominant concentrations and overall size structure roughly align with crab of instars X to XII for both sexes identified from other areas. An assumption that instar sizes from the eastern seaboard of the Untied States align with those in our study population is strengthened by no obvious growth pattern changes or relationships coinciding with increasing temperatures occurring in our study area.
Green crab population demographics
Green crab are known to exhibit a high degree of spatiotemporal variability in growth and reproductive patterns (Souza et al., 2011). Our samples of green crab were dominated by small crab and fresh shells, suggesting we were collecting young, frequently molting crab. In Maine, Berrill (1982) reported that crab settled in late summer to mid fall and grew to 5.5 mm CW by their first winter and 13–25 mm CW by their second winter, with sexual maturation thereafter at 2–3 years old. In adjacent Placentia Bay (NL), Best et al. (2017) estimated males and females to mature at 32 mm and 37 mm CW respectively and to spawn in June–July.
If patterns held, the dominant 20–25 mm CW concentration we observed in all three years of our samples would be expected to be crab in their second year of life. By extension, the secondary and higher level concentrations observed in 2024 would be expected to be both older crab and those large enough to be sexually mature. Like many crab species, female green crab molt before mating, thus lack of fresh shells in beach samples in July-August would suggest mating is not taking place in late summer or early fall. This would align with observations of Best et al. (2017) who determined females in Placentia Bay spawned during June–July, with August and September representing a period when recovery of spent females is common. The reproductive cycle was thought to be annual, with copulation typical in spring. Intuitively, our observations would appear to suggest that the North Harbour estuary population of green crab has similar life history dynamics as those in neighbouring Placentia Bay. Indeed, green crab found near the mouth of St. Mary’s Bay have been determined to be genetically akin to those in Placentia Bay (Lehnert et al., 2018).
Despite a broadening of size structure progressively occurring in the green crab population, the relationship of CW to CL held across crab sizes, with CW being roughly 30% higher than CL in most crab and following a linear relationship across the size spectrum. This finding closely reflects observations of the species for sixteen reports from areas of the Pacific and Northeast Atlantic oceans (Clark et al., 2001) as well as the US eastern seaboard and Nova Scotia (Squires, 1990). Accordingly, despite plasticity differences in life history dynamics to adapt to the subarctic conditions in Newfoundland versus in other green crab populations (Best et al., 2017), our data determine that shell morphometrics are the same.
Summary
Citizen science can be a convenient way to collect meaningful data on marine populations without need for extensive equipment or high capital investments. Herein, using a common tape measure and a simple visual shell aging index, we are able to provide novel insights into rock crab and green crab biology in Newfoundland. Our findings show that green crab invasions into existing rock crab habitats can be rapid and document basic rock crab life history processes in Newfoundland waters. We infer that St. Mary’s Bay rock crab have spring and summer molting periods, undertake deep to shallow water migrations, and have instar size structure and shell morphometrics akin to other Northwest Atlantic populations. For green crab, our data infer a June–July spawning period and similar size structure to the neighbouring Placentia Bay green crab populations, and confirm near-identical shell morphometrics to most Pacific and Atlantic stocks. For both species, allometric growth relationships remained constant over the study period with no obvious differentiation influenced by warming. Overall, these results highlight that despite occupying unique northern and subarctic habitats, life history attributes of these two brachyuryan crab species are similar to those occurring in more temperate areas of their expansive ranges. Such citizen science initiatives can fulfill a key need to document changes in speciation and biology within poorly monitored marine ecosystems as warming ensues.
Acknowledgements
We would like to thank Suzanne and Callum for helping with shell collections and Dr. Krista Baker and Kyle Matheson for reviewing our paper prior to submission.
References
Belair, M. C., and Miron, G. 2009. Predation behaviour of Cancer irroratus and Carcinus maenas during conspecific and heterospecific challenges. Aquatic Biology, 6: 41–49. https://doi.org/10.3354/ab00166
Berrill, M. 1982. The life cycle of the green crab Carcinus maenas at the northern end of its range. Journal of Crustacean Biology, 2: 31–39. https://doi.org/10.2307/1548108
Best, K., McKenzie, C. H., and Couturier, C., 2017. Reproductive biology of an invasive population of European green crab, Carcinus maenas, in Placentia Bay, Newfoundland Management of Biological Invasions, 8: 247–255. https://doi.org/10.3391/mbi.2017.8.2.12
Bigford, T. E. 1979. Synopsis of Biological Data on the Rock Crab, Cancer irroratus Say. NOAA Technical Report NMFS Circular 426. 26 p. https://doi.org/10.5962/bhl.title.63256
Blakeslee, A. M. H., McKenzie, C. H., Darling, J. A., Byers, J. E., Pringle, J. M., and Roman, J. 2010. A hitchhiker’s guide to the Maritimes: anthropogenic transport facilities long-distance dispersal of an invasive marine crab to Newfoundland. Diversity and Distributions, 16: 879–891. https://doi.org/10.1111/j.1472-4642.2010.00703.x
Clark, P. F., Neale, M., and Rainbow, P., S. 2001. A Morphometric Analysis of Regional Variation in Carcinus(Brachyura: Portunidae: Carcininae) with Particular Reference to the Status of the Two Species C. Maenasand C. Aestuarii.Journal of Crustacean Biology,21: 288–303.https://doi.org/10.1651/0278-0372(2001)021[0288:AMAORV]2.0.CO;2
DFO, 2025a. Rock crab (Cancer irroratus) Newfoundland and Labrador Region. https://www.dfo-mpo.gc.ca/fisheries-peches/ifmp-gmp/rock-crab-commun/2022/index-eng.html#toc1.0
DFO, 2025b. European Green Crab in Newfoundland Waters. https://www.dfo-mpo.gc.ca/species-especes/publications/ais-eae/greencrab-crabevert/index-eng.html
Gendron, L. 2001. Rock Crab of the Inshore Waters of Quebec: DFO, Atlantic Fisheries, SSR C4-02. http://www.qc.dfo-mpo.gc.ca/iml/en/produits/rapport.htm.
Gíslason, S., Pálsson, S., Jónas, P., J., Hermann, D., G., Jörundur, S., and Halldór, P., H. 2021. Population dynamics of three brachyuran crab species (Decapoda) in Icelandic waters: impact of recent colonization of the Atlantic rock crab (Cancer irroratus, ICES Journal of Marine Science, 78: 534–544. https://doi.org/10.1093/icesjms/fsaa059
Jeffery, N. W., DiBacco, C., Mallory, V., W., Lorraine, C., Hamilton, R., R., Stanley, R., E., Bernier, R., FitzGerald, J., Matheson, K., McKenzie, C., H., Ravindran, P., N., Beiko, R., and Bradbury, I., R. 2017. RAD sequencing reveals genomewide divergence between independent invasions of the European green crab (Carcinus maenas) in the Northwest Atlantic. Ecology and Evolution, 7: 2513–2524. https://doi.org/10.1002/ece3.2872
Kingsley, J. S. 1879. On a collection of Crustacea from Virginia, North Carolina, and Florida, with a revision of the genera of Crangonidae and Palaemonidae.,Proceedings of the Academy of Natural Sciences of Philadelphia,31: 383-427.
Klassen, G., and Locke, A. 2007. A biological synopsis of the European green crab, Carcinus maenas. Canadian Manuscript Report of Fisheries and Aquatic Sciences, no. 2818. 75 pp.
Lehnert, S. J., DiBacco, C., Jeffery, N. W., Blakeslee, A. M. H., Isaksson, J., Roman, J., Wringe, B. F., Stanley, R. R. E., Matheson, K., McKenzie, C. H., Hamilton, L. C., and Bradbury, I. R. 2018. Temporal dynamics of genetic clines of invasive European green crab (Carcinus maenas) in eastern North America. Evolutionary Applications, 11: 1656–1670. https://doi.org/10.1111/eva.12657
Matheson, K., and Gagnon, P. 2012. Effects of temperature, body size, and chela loss on competition for a limited food resource between indigenous rock crab (Cancer irroratus Say) and recently introduced green crab (Carcinus maenas L.). Journal of Experimental Marine Biology and Ecology, 428: 49–56. https://doi.org/10.1016/j.jembe.2012.06.003
OMGOR. 2025. Operational Mercator Global Ocean Ranalysis. E.U. Copernicus Marine Service Information (CMEMS). Marine Data Store (MDS). https://doi.org/10.48670/moi-00016
Page, K. 2002.“Cancer irroratus” (On-line), Animal Diversity Web. Accessed May 8, 2025 athttps://animaldiversity.org/accounts/Cancer_irroratus/
Rebach, S. 1987. Entrainment of Seasonal and Nonseasonal Rhythms by the Rock Crab Cancer irroratus, Journal of Crustacean Biology, 7: Pages 581–594. https://doi.org/10.1163/193724087X00360
Rossong, M. A. 2016. Impacts of newly established non-indigenous green crab (Carcinus maenas) on native fauna in Placentia Bay, Newfoundland. Doctoral (PhD) thesis, Memorial University of Newfoundland.
Souza, A. T., Ilarri, M. I., Campos, J., Marques, J., C., and Martins, I. 2011. Differences in the neighborhood: Structural variations in the carapace of shore crabsCarcinus maenas (Decapoda: Portunidae). Estuarine, Coastal and Shelf Science, 95: 424–430. https://doi.org/10.1016/j.ecss.2011.06.021
Squires, H.J. 1990. Decapod Crustacea of the Atlantic Coast of Canada. Canadian Bulletin of Fisheries and Aquatic Science, 221: 532 p.
Therriault, T. W., Herborg, L. M., Locke, A., and McKindsey, C. W. 2008. Risk Assessment for European green crab (Carcinus maenas) in Canadian Waters. DFO CSAS Res. Doc. 2008/042.
Young, A. M., and Elliott, J. A. 2020. “Life History and Population Dynamics of Green Crabs (Carcinus maenas”Fishes, 5: 4.https://doi.org/10.3390/fishes5010004
Citation: Mullowney, D.R.J., and Mullowney, M.J.E. 2025. Dynamics of indigenous rock crab (
Cancer irroratus) and invasive Green Crab (
Carcinus maenas) populations during invasion of a southern Newfoundland estuary.
J. Northw. Atl. Fish. Sci.,
56. 1–10. https://doi.org/10.2960/J.v56.m754