J. Northw. Atl. Fish. Sci., Vol. 53: 1–18
Publication (Upload) date: 19 Apr. 2022
*1Haugen, J.B. , 2Skomal, G.B., 3Curtis, T.H.
, 2Skomal, G.B., 3Curtis, T.H. , and 1Cadrin, S.X.
, and 1Cadrin, S.X.

1University of Massachusetts Dartmouth School for Marine Science and Technology,
836 South Rodney French Blvd, New Bedford, MA 02744.
*Corresponding author: jannebhaugen@gmail.com
scadrin@umassd.edu
2Massachusetts Division of Marine Fisheries, 836 South Rodney French Blvd, New Bedford, MA 02744.
gregory.skomal@mass.gov
3National Marine Fisheries Service, Atlantic Highly Migratory Species Management Division,
55 Great Republic Drive, Gloucester, MA 01930. tobey.curtis@noaa.gov
Haugen, J.B., Skomal, G.B., Curtis, T.H., and Cadrin, S.X. 2022. Interdisciplinary stock identification of North Atlantic porbeagle (Lamna nasus). J. Northw. Atl. Fish. Sci., 53: 1–18. https://doi.org/10.2960/J.v53.m732
Abstract
We conducted an interdisciplinary review of available information (i.e., genetics, life-history, and movement) to evaluate the stock structure of a previously targeted shark species, the porbeagle (Lamna nasus), in the North Atlantic. Most available information supports the conclusion that porbeagle consist of a single genetic population in the North Atlantic, which is relevant for determining species conservation status. However, the observed movement rates between the Northwest, Northeast Atlantic, and the Mediterranean appear to be low enough to consider separate spatial units for stock assessment and fishery management. The review reveals different interpretations among the organizations involved with the conservation, management, and assessments of porbeagle in the North Atlantic regarding biological population and stock boundaries. Differences in the spatial definition of management units among management organizations may pose an impediment to conserving porbeagle populations and achieving management objectives. We recommend an increased collaboration between organizations involved in highly migratory shark species as it would be beneficial for data collection, data inclusiveness, the robustness of assessments, and provide clarity for fishery managers, scientists, and the public on stocks and status. This review demonstrates that the interdisciplinary approach to stock identification is particularly valuable for data-limited species because no single approach typically has enough information to be definitive. Clearly defining management units that reflect the biological populations of porbeagle in the North Atlantic is expected to reduce uncertainty in stock assessments and help achieve current management and conservation goals of rebuilding North Atlantic porbeagle stocks.
Keywords: data-limited species; fisheries management; highly migratory species; regional fisheries management organizations; stock assessment; shark conservation
PDF
Download Citation Data

Citation to clipboard
 Reference management software (Endnote, Mendeley, RefWords, Zotero & most other reference management software)
Reference management software (Endnote, Mendeley, RefWords, Zotero & most other reference management software)
LaTex, BibDesk & other specific software
Introduction
Stock assessments inform fisheries management and conservation of threatened populations, but conventional stock assessment models assume the identification of self-sustaining populations that have negligible connectivity with other populations (Eagle et al., 2008). Therefore, differences between population boundaries and spatial management units can pose a problem for achieving management objectives (Kerr et al., 2017). For fisheries management purposes, stocks are considered discrete units, and each stock can be exploited independently (Cadrin et al., 2014). One main assumption in a stock assessment is that the assessed stock is a closed population with little to no emigration from or immigration into the stock area (Hilborn and Walters, 1992). If this assumption is violated, the model results may be less accurate (Begg, 2004; Cope and Punt, 2011; Punt et al., 2015; Goethel et al,. 2016; Jardim et al., 2018) and these inaccuracies have led to several fishery management failures (Cadrin, 2020). Stock identification examines the unit stock assumption used in these assessments, which is an important aspect of any stock assessment.
Stock identification also plays an important role in species conservation. Conservation of threatened species requires the identification of biological populations, which are more precisely defined using an ecological paradigm (e.g., “a group of individuals sufficiently isolated that immigration does not substantially affect the population dynamics or extinction risk over a 100-year time frame”) (Hanski and Gilpin, 1996) or an evolutionary paradigm (e.g., “a group of interbreeding individuals that exist together in time and space”) (Hedrick, 2000; Waples and Gaggiotti, 2006). To evaluate species conservation status and extinction risk, it is necessary to account for all population components so that each can be conserved and recovered (Ryman et al., 1995) while identifying any components that are ‘evolutionarily significant,’ such that their loss would be a permanent reduction in biodiversity (Waples, 1995). For example, stock identity is important for a species, or “distinct population segment,” being listed under the U.S. Endangered Species Act (Eagle et al., 2008).
Managing highly migratory species is challenging because of their broad-scale movements across international jurisdictions (Campana, 2016; Harrison et al., 2018). Due to the highly migratory behavior of many sharks, the identification of stock boundaries and the amount of mixing between adjacent stocks are important aspects to assure accurate assessments and management. However, stock identification of fish stocks and the exploration of alternative stock structures have often been historically ignored in stock assessments for teleost fish (Cadrin, et al., 2014), and elasmobranchs and other data-limited species (Hammer and Zimmerman, 2014).
The interdisciplinary assessment of life-history traits, environmental signals, genetic analyses, and movement studies has been successfully used for several species (e.g., Atlantic horse mackerel, Trachurus trachurus, Abaunza et al., 2008; beaked redfish, Sebastes mentella, Cadrin et al., 2010; yellowtail flounder, Limanada ferruginea, Cadrin 2010; winter flounder, Pseudopleuronectes americanus, DeCelles and Cadrin, 2011; Atlantic cod, Gadus morhua, Zemeckis et al., 2014). The use of multiple data sources can be complementary and provides more certainty in the results. This is particularly important for data-limited elasmobranch species in which stock identity is typically based on simple life history characteristics and spatio-temporal information from fishery statistics (e.g., seasonal and geographic patterns in landings; Begg, 2004). Results from these data-limited approaches can then help re-design future research for more definitive inferences of stock identity.
Although stock structure of porbeagle (Lamna nasus) has previously been examined (ICCAT/ICES, 2009; ICCAT, 2020; Curtis et al., 2016), an interdisciplinary assessment of porbeagle can provide more holistic information about intraspecific stock structure and boundaries as there are still discrepancies of stock boundaries, biological populations, and management units between government and non-governmental organizations for stock assessment, fishery management and species conservation (Table 1). The porbeagle is distributed across the North Atlantic Ocean and Mediterranean Sea (Fig. 1). The distribution is considered to be continuous across the North Atlantic, with a discrete distribution in the southern hemisphere (Compagno, 2001; Semba et al., 2013; Curtis et al., 2016). Porbeagle is commonly found on continental shelves and shelf edges in relatively cold waters (<18˚C) (Campana et al., 2002a; Skomal et al., 2021). Porbeagles were first targeted by fisheries in the Northeast Atlantic in the 1920s, but the fishery was closed in 2010 due to overfishing (ICCAT/ICES, 2009). The much smaller Northwest Atlantic porbeagle fishery started in 1961, and both stocks were considered overfished in the 2009 and 2020 stock assessments (ICCAT/ICES, 2009). Few porbeagles have been caught in the Mediterranean (Ferretti et al., 2008) and Mediterranean data are not included in porbeagle stock assessments, nor is there a separate stock assessment for Mediterranean porbeagle (ICCAT, 2020; Fig. 2)
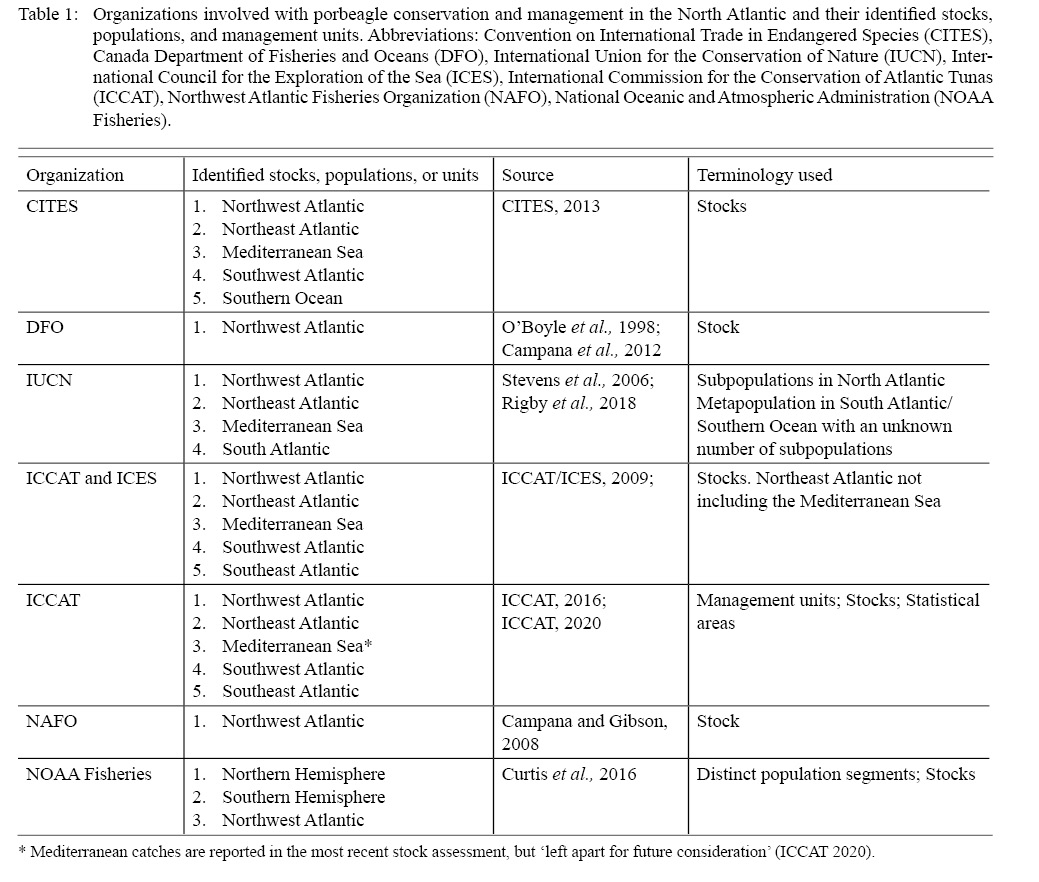
Table 1
As is typical of highly migratory species management, there are multiple national and international organizations, countries, and regions that take part in fishery management and conservation of porbeagle (Table 1). The International Council for the Exploration of the Sea (ICES) provides management advice on porbeagle fisheries in the Northeast Atlantic (excluding the Mediterranean), whereas the International Commission for the Conservation of Atlantic Tunas (ICCAT) collects data, reports annual catches, conducts stock assessments, and provides advice on tuna and tuna-like fisheries where porbeagle are caught in the entire North Atlantic and Mediterranean. The Northwest Atlantic Fisheries Organization (NAFO), a Regional Fisheries Management Organization (RFMO), is not regularly involved in the management of Northwest Atlantic porbeagle fisheries but has contributed with advice upon request in the past (Campana and Gibson, 2008). The Canada Department of Fisheries and Oceans (DFO) manages porbeagle fisheries in Canadian waters, and the U.S. National Marine Fisheries Service (NMFS) manages porbeagle fisheries in the United States. Northeast Atlantic fisheries are managed by the European Common Fisheries Policy in the European Union (E.U.) and by individual non-EU countries. The Northeast Atlantic Fisheries Commission (NEAFC) is the RFMO for the Northeast Atlantic, and the General Fisheries Commission for the Mediterranean (GFCM) is the RFMO for the Mediterranean. In addition to these management organizations, there are several conservation organizations and treaties that are involved in the management and conservation of highly migratory sharks in the North Atlantic, and several nations have independent mandates for species conservation. There is presently little collaboration among management organizations, conservation organizations, and countries within regional organizations, and fisheries management varies greatly among European countries and between the U.S. and Canada (Campana, 2016; Cameron et al., 2019). There are several examples in the last 20 years where management advice, management actions, and conservation actions for the same stocks have been uncoordinated and, in some cases, contradicting (Fig. 3).
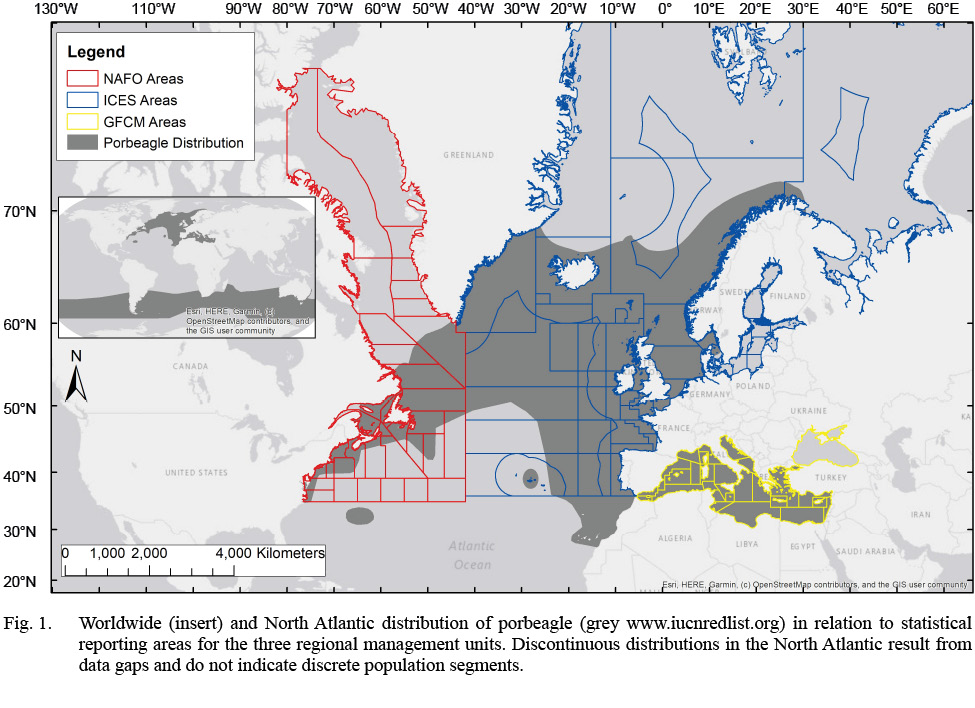
Fig. 1
Different stock definitions and boundaries among fishery management or conservation organizations can hinder the success of fishery management and the ability to achieve conservation goals. Therefore, refining the terminology and finding agreement on stock boundaries among parties for the North Atlantic porbeagle is expected to improve collaboration among parties and increase the effectiveness of fishery management, the performance of stock assessments and conservation assessments of this overexploited, highly migratory species. The objectives of this study were to 1) provide a comprehensive review of the information available on North Atlantic porbeagle stock structure using diverse stock identification approaches; 2) evaluate if current stock delineations reflect biological populations; and 3) consider the extent to which the misalignment of stock identification could impact stock assessments, conservation assessments, and management.
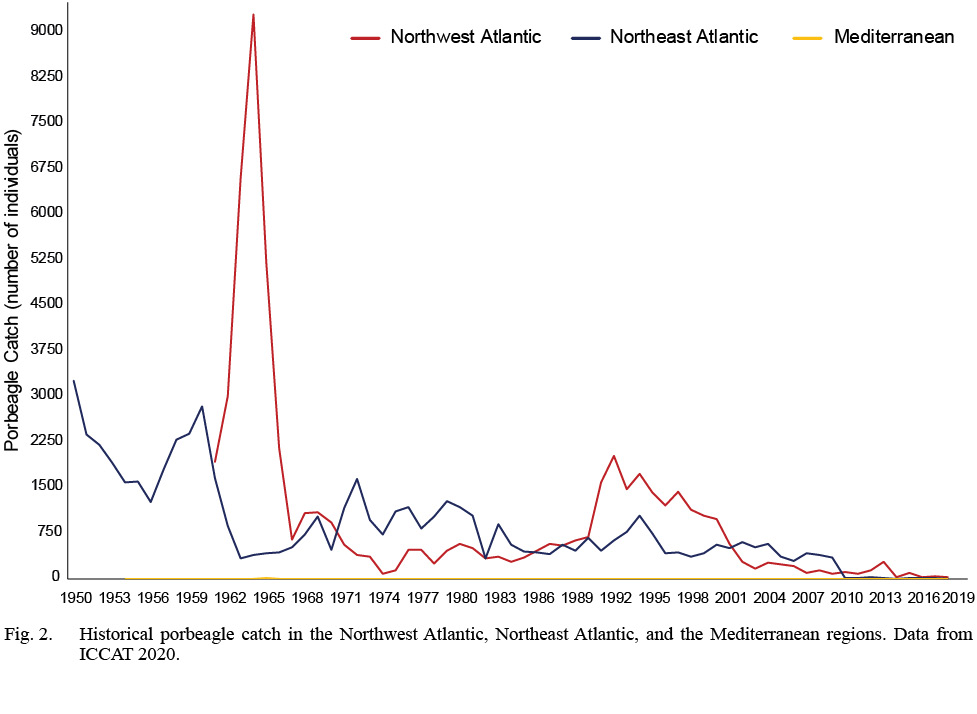
Fig. 2
Porbeagle Stock Identification Information
Genetic analyses
The investigation of genetic diversity within and among populations is valuable for species with depleted abundance like the porbeagle (Testerman, 2014). Both nuclear DNA and mitochondrial DNA analyses can be useful molecular techniques to identify stock structure (Antoniou and Magoulas, 2014; Mariani and Bekkevold, 2014). The population structure of porbeagle was investigated in the North and South Atlantic using the nucleotide sequence of mitochondrial DNA by (Kitamura and Matsunaga, 2010). Based on four sharks sampled off Nova Scotia and 49 sampled in the South Atlantic from 1992–2007, the authors found greater nucleotide diversity in the Nova Scotian samples. They attributed these findings to larger effective population size or different breeding populations in the North Atlantic (e.g., Northwest and Northeast Atlantic breeding populations), noting that some of the tissue samples were >20 years old and porbeagle abundance was greater in the 1990s than when the samples were analyzed (Kitamura and Matsunaga, 2010). Kitamura and Matsunaga (2010) concluded that the gene flow of porbeagles between the North Atlantic and South Atlantic is restricted, and conservation efforts should consider them to be separate populations. However, with samples only in the Northwest Atlantic, this study could not infer any stock structure in the North Atlantic.
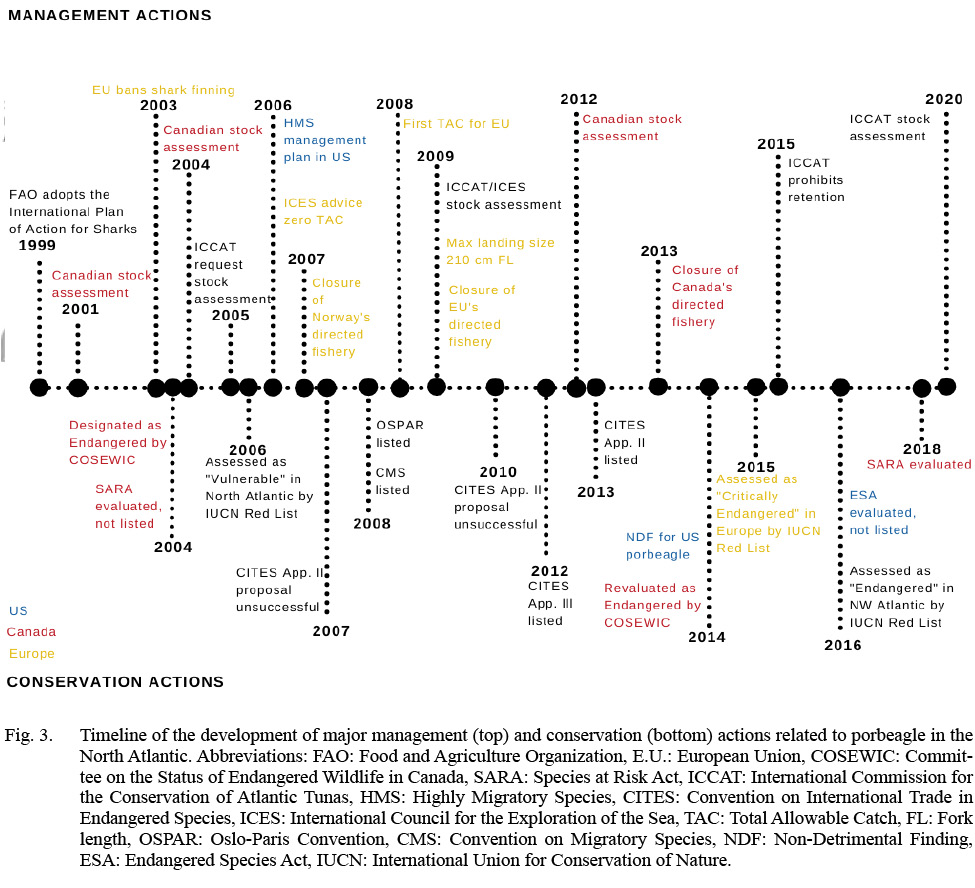
Fig. 3
The genetic structure of porbeagles from the Northern and Southern hemispheres, including genetic differences within the North Atlantic, was further investigated by (Testerman, 2014). This study used a mitochondrial control region from 224 porbeagles collected at one site in the Northwest Atlantic (n = 40; off Canada), two sites in the Northeast Atlantic (n = 35; off Denmark and the United Kingdom), and five sites in the Southern hemisphere (n = 149; off Chile, the Falkland Islands, South Africa, Tasmania, and New Zealand). Similar to the results of Kitamura and Matsunaga (2010), Testerman (2014) found significant genetic differences and no genetic connectivity between the northern and southern hemispheres, and the results represented the greatest intraspecific genetic diversity for any shark species measured to date. Temperature preferences of porbeagle sharks (<18°C; Skomal et al., 2021) suggest that equatorial seas are too warm for porbeagles, thereby forming a barrier between the hemispheres (Kitamura and Matsunaga, 2010; Testerman, 2014), although recent evidence suggests porbeagles may occupy equatorial waters (ICCAT, 2020). Other shark species that predominantly inhabit temperate waters transit the equator via deep tropical waters (e.g., basking shark; Skomal et al., 2009), genetic analyses from two studies using different genetic markers, when coupled with existing distribution and movement data (see below), suggest that porbeagle movement may be limited by warm equatorial surface waters.
Testerman (2014) also found no genetic differences between porbeagles sampled from the Northwest and Northeast Atlantic. Assuming a 13-year generation time, reproductive mixing by 2–12 migrating porbeagle sharks between the Northwest and Northeast Atlantic every year, or 30–150 migrants per generation, is sufficient gene flow for genetic structure to be of similar character (Testerman, 2014). A recent and more comprehensive analysis of mitochondrial DNA confirms two separate populations in the North Atlantic and southern hemisphere and no genetic structure within the North Atlantic (n = 70 northwest Atlantic, n = 99 northeast Atlantic, n = 2 Mediterranean markets; González et al., 2021).
These results are indicative of an anti-equatorial distribution with genetically divergent northern and southern hemisphere stocks (Kitamura and Matsunaga, 2010; Testerman, 2014; González et al., 2021). No genetic differences between porbeagles sampled in the Northwest and Northeast Atlantic have been found, but the genetic structure of this species in the Mediterranean is based on only two market samples with an unknown location of capture. There appears to be enough reproductive mixing across the Atlantic to promote gene flow, and the lack of genetic structure suggests that Northwest and Northeast Atlantic porbeagle are not reproductively isolated and may form a single evolutionary significant unit. However, a much greater mixing rate between the east and west might be needed to replenish depleted stocks (Waples, 1998). To improve the certainty of stock structure in the North Atlantic, genetic studies of porbeagle with samples from Canada and the U.S. in the Northwest Atlantic, in addition to samples from various locations in the Northeast Atlantic and the Mediterranean, should be analyzed in conjunction with other stock identification methods (i.e., biological, conventional, and electronic tags).
Life-history traits
Life-history traits are one of the oldest tools used to inform management of a fishery resource, largely because they are relatively inexpensive and easy to sample (Begg, 2004). Therefore, life history attributes are generally the most accessible and robust information for data-limited species such as the porbeagle. The three groupings of life-history parameters that are commonly used for stock identification are 1) age, size, growth, and mortality; 2) reproduction, maturity, fecundity, and recruitment; and 3) spatial distribution and abundance (McBride, 2014). Many of these traits are routinely sampled for stock assessment and are available for stock identification. Life-history traits are phenotypic, so geographic variation can reflect genetic or environmental differences.
Growth and maturity
Although porbeagles are distributed across the North Atlantic, information on life-history traits varies greatly between regions. The Northwest Atlantic is relatively well-sampled, and there are some samples from the Northeast Atlantic but few samples from the Mediterranean (Table 2). Validated growth studies have been published for porbeagle in the Northwest Atlantic, but there are no comparable studies in the Northeast Atlantic. The former comprises two studies that sampled porbeagle from U.S. and Canadian waters, including lengths and vertebrae for age determination. Aasen (1963) sampled porbeagles in 1961 from Georges Bank, the Gulf of Maine, the eastern Scotian Shelf, and St. Pierre Bank, and Natanson et al. (2002) sampled between 1966 and 1999 from Massachusetts to the Grand Banks. Their growth estimates were similar (e.g., estimates of asymptotic length were not significantly different). Vertebral band pair counts produced maximum ages of 25 and 24 years for males and females, respectively (Natanson et al., 2002), which are similar to the maximum age estimated using bomb radiocarbon (26 years, Campana et al., (2002b)). However, these could represent minimum estimates of longevity because of uncertainties in vertebral aging techniques (Natanson et al., 2018); calculated longevity estimates were as high as 46 years in an unfished population (Natanson et al., 2002).
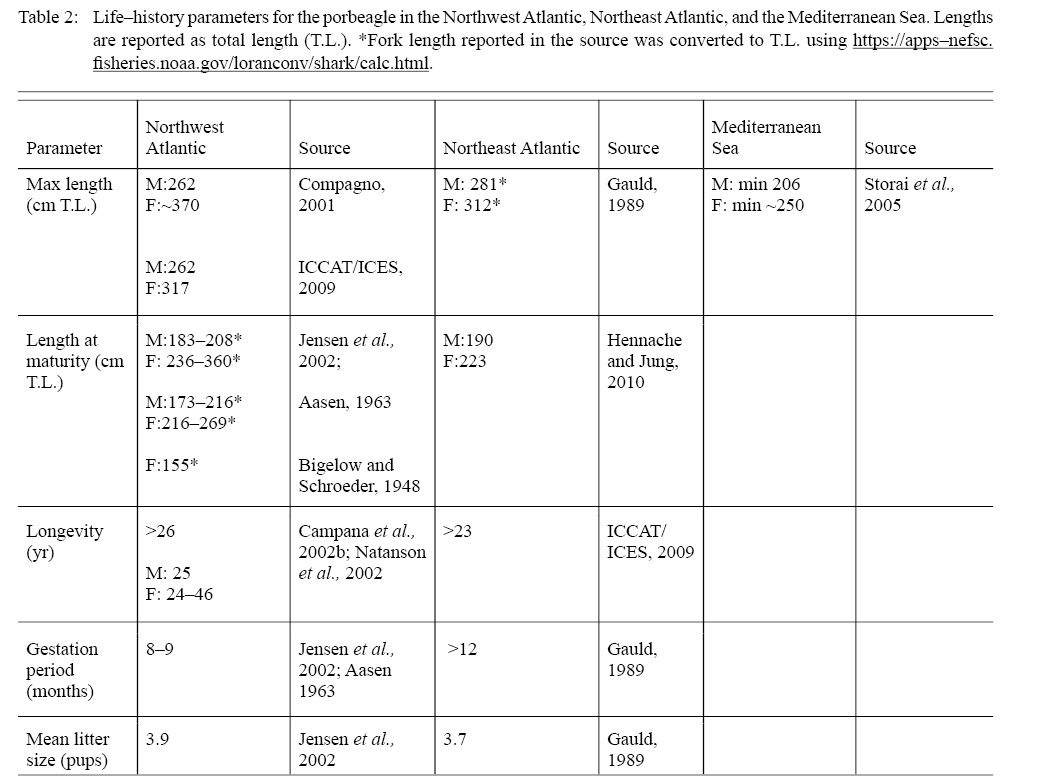
Table 2
Another metric that stock assessments rely on is size or age at maturity. The length at maturity for porbeagles in the Northwest Atlantic ranges from 173 to 216 cm total length (T.L.) for males (Aasen 1963; Jensen et al., 2002) and 155 to 269 cm T.L. for females (Bigelow and Schroeder, 1948; Aasen 1963; Jensen et al., 2002). The estimated size at 50% maturity of Canadian and U.S. porbeagle mature is 198 cm T.L. and 246 cm T.L. for males and females, respectively (Jensen et al., 2002). Based on fewer observations, the porbeagle in the Northeast Atlantic matures at smaller sizes and reaches a smaller maximum size than those in the west (Table 2), and length at 50% maturity is 191 cm T.L. for males and 226 cm T.L. for females (Hennache and Jung, 2010). Age at maturity for porbeagle in the Northwest Atlantic is 13 years for females and 8 years for males (Jensen et al., 2002; Natanson et al., 2002), but there are no age estimates for comparison in the Northeast Atlantic.
Sex ratios
The reported sex ratios of porbeagle in the three regions of the North Atlantic were statistically tested for significant deviances from 1:1 the sex ratios using a G-test (Sokal and Rohlf, 2012). The proportion of males has been reported as 0.46 in the Northwest Atlantic (n = 122, Aasen, 1963), 0.43 in the Northeast Atlantic (n = 1368, Gauld, 1989), and 0.33 in the Mediterranean (n = 15, Storai et al., 2005). Based on these data, all areas have female-biased sex ratios that significantly deviate from the expected 1:1 male: female ratio (P < 0.01). However, no significant differences were found among the three areas (P = 0.25). The Mediterranean has a low sample size compared to the other areas, but excluding the Mediterranean did not result in significant differences in sex ratios between the Northeast and Northwest (P = 0.28). Despite the significant female-bias in sex ratios within each population, the result may not be ecologically significant because the differences are relatively small. Further, as the data are fisheries-dependent, the detectable differences could be biased by the effort deployed, time of year, or area of operation of the fishery.
Although these results suggest that all regions in the North Atlantic (Northwest, Northeast, and Mediterranean) have had sex ratios favoring female sharks, a more recent estimate in the Northeast Atlantic indicates that the overall male to female sex ratio has increased to 0.84 (Hennache and Jung, 2010). Male bias was apparent in certain locations, like the St. Georges Channel (male: female 0.84) in the Irish Sea and north in the Gulf of Gascogne (male: female 0.85). However, in the waters off the southern tip of Ireland, there were fewer females than males, with a sex ratio of 1.35 (Hennache and Jung, 2010). These areas are in close proximity, and the difference in sex ratios can be influenced by time of year and environmental conditions, particularly in conjunction with parturition. Porbeagles segregate by size and sex (Aasen, 1963; Compagno, 2001; Natanson et al., 2019), so differences among sex ratio samples may not represent the population. Instead, these differences at specific locations can help identify important nursery areas in conjunction with movement studies.
Mating, gestation, and nursery areas
In the Northwest Atlantic, mating is thought to occur from September to December, the gestation period is estimated to be eight to nine months (Aasen, 1963; Jensen et al., 2002), and parturition occurs between April and June (Aasen, 1963; O’Boyle et al., 1998; Jensen et al., 2002). Natanson et al. (2019) suggested that porbeagles have a biennial instead of an annual reproductive cycle based on the examination of ovaries, which were unlikely to be ready for a new litter in the fall after giving birth in the spring of the same year. In the Northeast Atlantic, Gauld (1989) inferred a mating season of December–January, a gestation period of >12 months based on two distinct size groups of embryos in females caught in December-February, and parturition in the summer/autumn. The mean number of embryos in each porbeagle litter was 3.7 pups in the Northeast Atlantic, which is similar to the reported mean litter size of 3.9 pups in the Northwest Atlantic (Gauld, 1989; Jensen et al., 2002).
Parturition and nursery areas for North Atlantic porbeagle are not well understood. However, investigations of site fidelity from tagging studies, the capture of gravid females, and changes in sex ratios have improved our knowledge of locations for potential biologically important areas in the North Atlantic. In the Northwest Atlantic, the observation of mature, ovulating, or gravid females and females with mating scars from September–December on the Scotian Shelf and Grand Banks suggests that these areas are mating grounds for the porbeagle (Jensen et al., 2002). Georges Bank has also been suggested as a mating ground-based on high catches of mature females in the summer (Campana et al., 2010). However, Natanson et al. (2019) noted, with regard to that study, that no males were caught, biological samples were not collected, and these mature females were part of a reproductively resting population on Georges Bank. The Sargasso Sea south of 35°N has been suggested to be a parturition area based on seasonal north-south migrations undertaken by large females in the summer (Campana et al., 2010). However, given the resting population of females in the Stellwagen/Georges Bank area and the lack of biological data, Natanson et al., (2019) concluded that females do not migrate to the Sargasso Sea for pupping because they were only assumed to be gravid and did not transit mating grounds before moving south. Previous studies (Bigelow and Schroeder, 1948; Jensen et al., 2002; Kohler et al., 2002) and ongoing research in the Gulf of Maine indicate that pregnant females remain in the Gulf of Maine, and young-of-the-year porbeagles spend approximately the first two years of their lives in that area, where they make offshore migrations to the Gulf Stream in the winter, and move inshore in the summer (Skomal et al., 2021; Anderson et al., 2021).
In the Northeast Atlantic, recent studies suggest that porbeagles may use temperate waters in the Bay of Biscay as a potential nursery ground (Saunders et al., 2011; Biais et al., 2017). In the Mediterranean, an examination of 33 porbeagle caught in Italian waters from 1871–2004 revealed both juveniles and mature porbeagles (Storai et al., 2005). The authors hypothesized that porbeagles do not reproduce in Italian waters but in other areas of the Mediterranean (Storai et al., 2005). However, the Adriatic Sea has been interpreted as an important mating area (southern-middle open waters of the Adriatic Sea), pupping area (middle of the Adriatic Sea), and nursery area (middle-northern Adriatic Sea) areas based on size, sex, and maturity of the captured porbeagles (Soldo, 2006; Scacco et al., 2012; Lipej et al., 2016). A 104 cm T.L. male porbeagle was recently caught in December by fishermen in the northern Adriatic Sea, the first record of this species in Slovenian waters (Lipej et al., 2016). These studies confirm the rarity of porbeagles in the Mediterranean but indicate that juvenile porbeagles are present in the Mediterranean throughout the year. Furthermore, the IUCN has suggested that the Mediterranean includes nursery areas for porbeagle with few adult sharks occupying the area year-round (Stevens et al., 2006; Rigby et al., 2018), and a recent review found no evidence that the Mediterranean porbeagle is isolated from the Northeast Atlantic (Curtis et al., 2016), inferring that there is likely connectivity between these two regions. However, additional research is needed to address the questions of connectivity in the Northeast/Mediterranean and potential nursery areas for porbeagle in the North Atlantic.
Shark nursery areas are thought to be driven by factors such as food availability and shelter from predators (Branstetter, 1990). Heupel et al. (2007) suggested the following quantifiable criteria to delineate such areas: 1) a higher density of sharks than the surrounding areas; 2) the sharks remain or return for an extended period; and 3) the area is used repeatedly across years. No study to date has quantified porbeagle nursery areas in the North Atlantic using the suggested criteria, which help separate nursery areas from other areas where juvenile sharks have been documented to occur. Although there are several studies showing site fidelity and large porbeagle aggregations (e.g., Pade et al., 2009; Biais et al., 2017; Haugen and Papastamatiou 2019), the occurrence of juvenile sharks or aggregating sharks in an area is not necessarily equivalent to a nursery area (Heupel et al., 2007).
Collectively, these studies suggest similar rates of growth and maturation among areas but some minor differences in other reproductive traits, such as mating season, gestation period, and parturition between the western and eastern regions in the North Atlantic. As suggested by Curtis et al. (2016), porbeagles appear to be relatively uncommon in the Mediterranean, and it may represent a fringe of its Northeast Atlantic range. Most studies on phenotypic traits of porbeagle in the North Atlantic have focused on the Northwest Atlantic, with a data gap in life-history studies for the remaining regions. The life history information available for porbeagles in U.S. and Canadian waters suggests no difference between the two areas, suggesting that U.S. and Canadian porbeagles are part of the same biological population. However, none of the studies have explicitly investigated differences between Canadian and U.S. porbeagle, and most studies pooled samples from both countries.
Differences in size at maturity can be affected by fishing pressure. Cassoff et al. (2007) estimated the age at maturity for Northwest Atlantic porbeagle to be 8–7 years for males and 19–14 years for females when the population was unfished (1961–1963). The size at maturity of males decreased (from 179–174 cm curved fork length) after large declines in abundance due to fishing, but there was no change in female size at maturity (216 cm curved fork length) (Cassoff et al., 2007). Therefore, morphological traits, such as size at a certain life stage, can vary between regions based on environmental conditions, like density dependence and selection. Although both sides of the Atlantic have experienced large declines in abundance, the Northeast Atlantic porbeagle has had much greater removals, which may explain some of the differences in growth between the east and west (ICCAT/ICES, 2009). The sample composition (e.g., size range), capture location, time of year of sample collection, and aging methodology can also affect the outcome when sample sizes are small. Research to examine the differences in the abundance, age, size, growth, mortality, reproduction, maturity, fecundity, and recruitment of porbeagle in the Northeast Atlantic and Mediterranean is needed.
Movement and tagging information
Recoveries of conventional tags and information from electronic tags can be used to empirically determine mixing between groups of fish and to investigate dispersal and residency patterns. For highly migratory species, acoustic and satellite telemetry tags are particularly useful to assess movement between stocks, but electronic tagging information has historically rarely been used for stock identification purposes (DeCelles and Zemeckis, 2014).
Both satellite archival and conventional tags have been used to investigate porbeagle movement in the North Atlantic. Collectively, the published literature has information retrieved from 322 porbeagles tagged from 1961–2020 (total tagged = 3044; Table 3). The NMFS Cooperative Shark Tagging Program provides porbeagle data from the longest-running conventional shark tagging program (Kohler and Turner, 2020). Of 1754 porbeagles tagged in the Northwest and Northeast Atlantic during 1962–2013, 178 (9.8%) fish were recaptured (Kohler and Turner 2020). Several porbeagles were recaptured in a different nation’s Exclusive Economic Zone than from where they were tagged (Fig. 4). The average tag deployment was three years, with a mean distance traveled of 424 km (Kohler and Turner, 2020). All of the conventional tagging studies conducted to date in the North Atlantic, representing 261 (8.8%) recaptures from 2971 tags, indicated site fidelity to the Atlantic region where the shark was tagged.
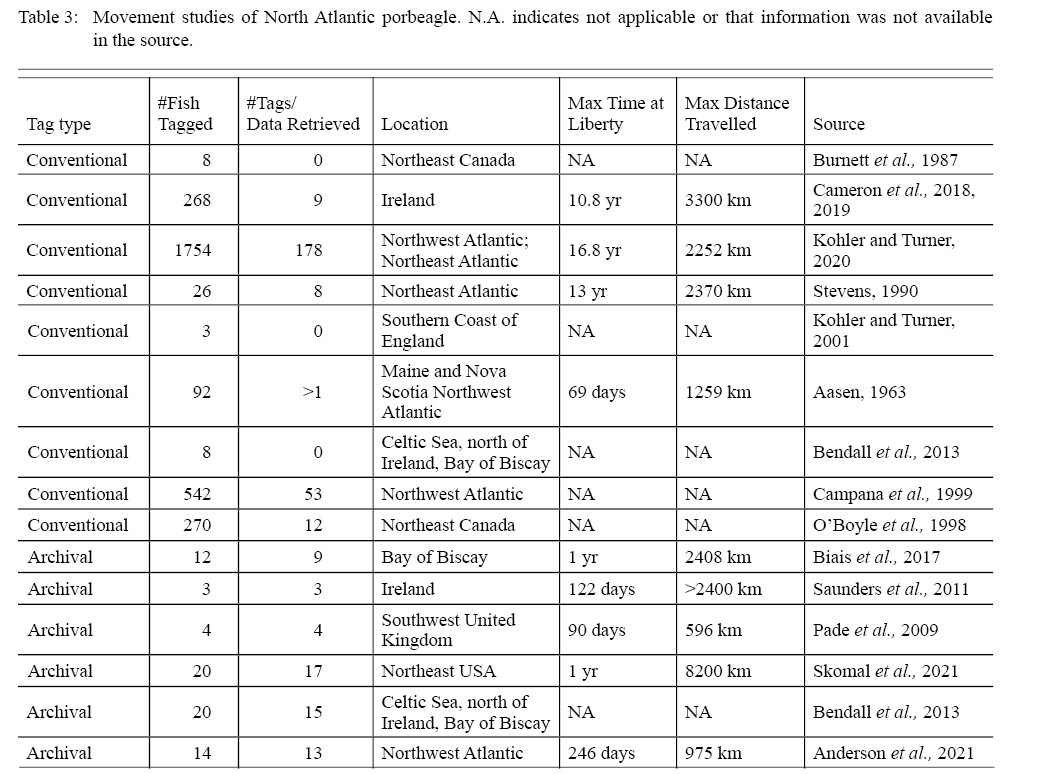
Table 3
Pop-up satellite archival transmitting (PSAT) tags have been used to investigate migratory routes and habitat preferences of porbeagle in the Northwest Atlantic. PSATs attached to 17 porbeagles on Georges Bank showed minimum linear movements ranging from 937 to 3310 km over track durations of 120 to 360 days; all of the sharks remained in the western North Atlantic from the Gulf of Maine, the Scotian Shelf, on George's Bank, and in the deep, oceanic waters off the continental shelf along the edge of, and within, the Gulf Stream (Skomal et al., 2021). The results indicated broad, seasonally-dependent vertical and horizontal movements of porbeagles in the Northwest Atlantic with most of their time (97%) in temperatures ranging from 6–20°C (Skomal et al., 2021). This study also showed that porbeagles move routinely from the U.S. Exclusive Economic Zone (EEZ) into Canadian waters and that they spend a significant amount of their time in the high seas outside of any EEZ (Kohler and Turner, 2020; Skomal et al., 2021; Fig. 5).
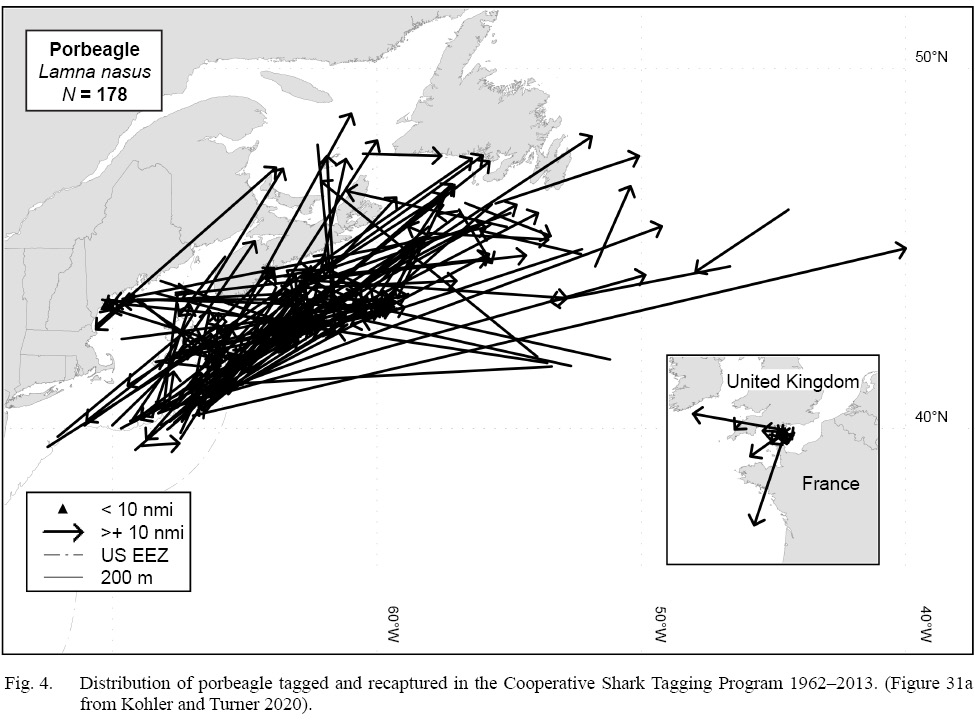
Fig. 4
The only documented transatlantic crossing by a porbeagle was a female shark conventionally tagged in Irish waters in 1972 and recaptured on the Grand Banks outside of Canada ten years later, a distance of approximately 3300 km (Cameron et al., 2018). The longest estimated travel distance for one shark in 365 days was 13–352 km (Biais et al., 2017). In the Northeast Atlantic, multiple studies suggested short-term residency in coastal waters (i.e., Celtic Sea, English Channel, Bay of Biscay) during the summer months and long-term site fidelity based on porbeagles returning to the same areas (Irish waters) for multiple years (Pade et al., 2009; Saunders et al., 2011; Biais et al., 2017; Cameron et al., 2019).
Tagging studies have also recorded porbeagle sharks moving near or past the current Northeast Atlantic jurisdictional boundaries. Out of nine sharks PSAT-tagged in the Bay of Biscay, one traveled south of the Northeast Atlantic stock border (36°N) to 33°N, while another tag popped off at 31°W close to the east/west boundary in the Atlantic (Biais et al., 2017). Bendall et al. (2013) tagged 14 porbeagle sharks with PSAT tags around the British Isles, where two sharks made extensive movements from the tagging locations. One shark migrated south towards the Strait of Gibraltar, and the other traveled far west into the central Atlantic Ocean. The tag popped up at ~40°W, only 2° east of the western border between the Northwest and Northeast Atlantic, the furthest a tagged porbeagle has traveled from the U.K. (Bendall et al., 2013). Out of three porbeagles (one male, two female) tagged Northwest of Ireland, the male porbeagle migrated south towards Morocco and past the southern boundary of the Northeast Atlantic management unit to 33°N (Saunders et al., 2011). The shark was between Morocco and Madeira when the tag popped off. The two female sharks spent most of their time on the continental shelf outside of Western Ireland near a high abundance of Atlantic mackerel (Scomber scombrus) in the region.
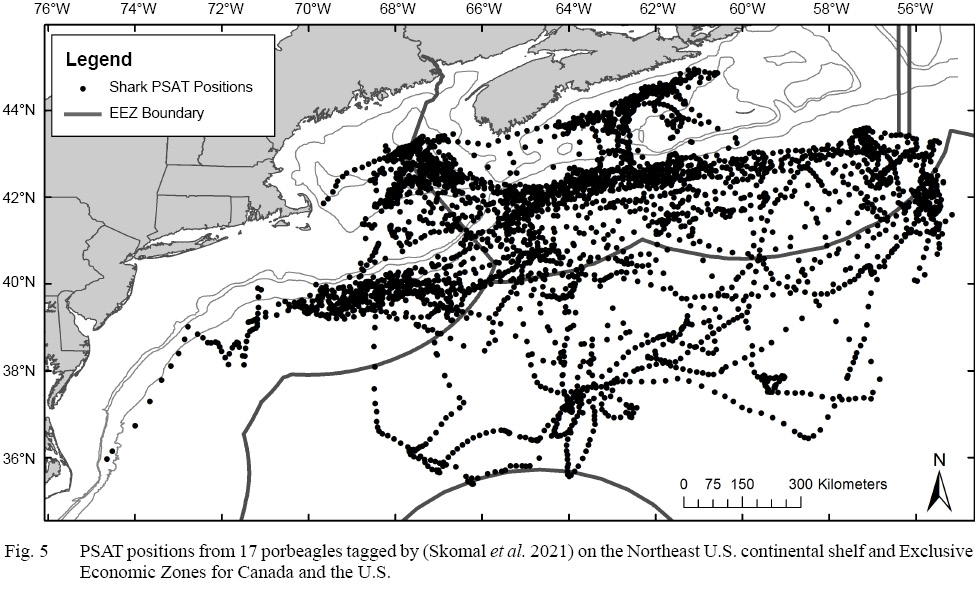
Fig. 5
Tagging studies confirm that porbeagles have large home ranges and are capable of long-distance migrations. Porbeagle distribution in the Northeast Atlantic ranges at least from the North Sea to Morocco and south of the entrance to the Mediterranean Sea, past the current management unit for the Northeast Atlantic porbeagle. Distribution in the Northwest Atlantic ranges from Canada to the Sargasso Sea, with several studies observing porbeagle movements between the U.S. and Canada. Information from tagging suggests a low rate of movement between the northeast and northwest Atlantic, with one porbeagle tagged in Irish waters and recaptured on the Grand Banks ten years later (Cameron et al., 2018) from a total of 346 conventional tag recaptures (ICES, 2022), and location estimates from several archival tag deployments that indicate movement across the ICES-NAFO boundary (42°W) from porbeagle tagged in the Bay of Biscay (ICES, 2022) and off the British Isles (ICES, 2022). There are no observed movements between the Northeast Atlantic and Mediterranean, and a thermal habitat barrier may limit movement between those areas (ICES, 2022). To further investigate the mixing potential between the Northeast Atlantic and the Mediterranean, fine-scale movement patterns between the waters outside of Northwest Africa and the Mediterranean should be investigated.
Discussion
Interdisciplinary synthesis
Best practices in stock identification involve an interdisciplinary synthesis of all available information to determine the most plausible paradigms of population structure in which information from different approaches is integrated conceptually and geographically (Begg, 2004; Cadrin et al., 2014). Information on distribution, movement, and geographic variation from advanced technologies can be reconciled with previous information from traditional methods to define spatial population structure. Considering all available information on life history, genetics, and movement of porbeagle in the North Atlantic, there appears to be a single genetic population of porbeagle in the Northern hemisphere, with a low frequency of transatlantic movements. However, low movement rates and some phenotypic differences between the Northwest Atlantic and the Northeast Atlantic suggest two semi-distinct stocks that are demographically independent on ecological timescales. Therefore, the U.S. definition of a single distinct population segment in the North Atlantic (Curtis et al., 2016) is consistent with the inference of a single genetic population. The Northeast Atlantic and Mediterranean management units defined by ICES/ICCAT, GFCM, CITES, and IUCN may not reflect the single biological population in the North Atlantic but may be appropriate for stock assessment and fishery management because of low mixing rates with the Northwest Atlantic areas. Similarly, the NAFO and Canadian definition of a Northwest Atlantic porbeagle stock (e.g., Campana and Gibson 2008; Campana et al., 2012) may be appropriate because of low mixing rates with the Northeast Atlantic.
The available information on porbeagle stock structure in the Northwest Atlantic indicates that U.S. and Canadian porbeagle are part of the same biological population. Life history traits suggest relatively homogeneous traits within the Northwest Atlantic and some differences with the Northeast Atlantic (e.g., gestation period; Table 2). Possible nursery grounds for this population are in U.S. waters (Georges Bank and the Gulf of Maine) and Canadian waters (Scotian shelf and Grand Bank region). Substantial movement across the U.S. EEZ from multiple studies (Kohler and Turner, 2020; Skomal et al., 2021), and no difference in life-history and genetics, indicates that porbeagles in the Northwest Atlantic are part of the same stock, as is currently assumed in NAFO, ICES, ICCAT, and Canadian stock assessments (Campana and Gibson, 2008; ICCAT/ICES, 2009; Campana et al., 2015). There is currently no evidence of subpopulation structure within the Northwest Atlantic, although U.S. and Canadian fisheries have been managed differently over time.
There is no strong evidence of the stock identity of the Mediterranean porbeagle. Porbeagles are rarely encountered in the Mediterranean, but the area is part of their distribution. Therefore, hypotheses about the most likely stock structure in the Northeast Atlantic and the Mediterranean need to be tested with more information on geographic variation and mixing. Based on this review, particularly the Mediterranean genetic samples and Curtis et al. (2016), we hypothesize that the Mediterranean porbeagle is not a separate population from the Northeast Atlantic stock. We found no evidence that the Mediterranean porbeagle is a separate biological population or stock, as evaluated by CITES (CITES, 2013), and there are no indicators that IUCN’s evaluation of the Mediterranean porbeagle as a subpopulation in the Northeast Atlantic is more appropriate. Observations of young of the year and mature sharks in the Mediterranean and the inference of a nursery ground for Northeast Atlantic porbeagle imply some connectivity between the areas (Storai et al., 2005; Soldo, 2006; Stevens et al., 2006; Scacco et al., 2012; Lipej et al., 2016). Since there is no evidence of a separate Mediterranean porbeagle stock, we argue that there is no population structure in the Mediterranean unless evidence of structure is presented. Therefore, we suggest one biological population of porbeagle in the Northeast Atlantic with some sharks utilizing the Mediterranean habitat. However, the lack of observed movements and apparent thermal habitat barrier between the Northeast Atlantic and the Mediterranean Sea may justify separate stock assessment units (ICES 2022).
Mixing between Northwest and Northeast Atlantic
Empirical evidence from movement and tagging studies suggests that Northwest and Northeast Atlantic porbeagle mixing rates are low. There is only one observation of an individual shark making a transatlantic crossing from Ireland to Canada (Cameron et al., 2018), a PSAT deployment from the British Isles suggested movement to the Flemish Cap (45°W; ICES, 2022), another PSAT tag deployed off Ireland in summer popped off in winter in the central Atlantic (Bendall et al., 2013), and several PSAT tags deployed in the Bay of Biscay and the Celtic Sea had winter positions in the central Atlantic (ICES, 2022). Despite the few observed transatlantic movements, the species is capable of broad latitudinal migrations, and the distance between Europe and North America is within their observed movements. Although most published movement studies for North Atlantic porbeagle provide evidence for the separation of Northwest and Northeast Atlantic stocks, sample sizes were relatively small, and tracking was too brief (up to one year) to make general inferences on a population level. Larger sample sizes of returned tags from conventionally tagged porbeagles and an increased number of satellite-tagged porbeagles may reveal more transatlantic crossings.
There is no genetic evidence of separate stocks in the Northwest and Northeast, and few individuals making the transatlantic crossing every year would be needed for sufficient gene flow between the two regions (Testerman, 2014). Although there have only been a few transatlantic movements documented, tagging studies cannot alone determine with certainty that reproductive isolation exists. These migration numbers are based on estimated longevity; therefore, changes in longevity will impact the number of individuals needed to make transatlantic migrations each year to have sufficient gene flow for there to not be genetic differentiation of porbeagles in the North Atlantic (Testerman, 2014). More importantly, the low estimate of individuals crossing the Atlantic and reproducing on the other side per year is not likely enough for recruitment, recolonization, or rebuilding within fishery management or conservation timelines.
Movement studies indicated that porbeagles are capable of movements over great distances (Table 3), but the scope of the current tagging studies appears to be too small to empirically identify the mixing that would support the estimated gene flow. One possible mechanism for gene flow between the Northwest and Northeast Atlantic could be the result of a shared pupping area in the central Atlantic with random recruits to either side of the Atlantic Ocean (Campana et al., 2010). However, this hypothesis requires further investigation, including improved delineation of parturition areas as well as movements of neonate porbeagles (Natanson et al., 2019). Due to uncertainty in pupping and nursery areas, low gene flow and recruitment, limited movement studies, and some demographic independence in phenotypic traits between the Northwest and Northeast Atlantic, the use of one eastern and one western stock of North Atlantic porbeagle is appropriate.
Historical, current, and future management
Perceptions of appropriate spatial units for species conservation or fishery management vary among organizations involved in fisheries management and conservation of highly migratory sharks in the North Atlantic. Communication among organizations and consideration of all available information is needed to achieve a holistic view of the complexity of managing highly migratory sharks in the North Atlantic. An interdisciplinary approach to assessing the stock structure of porbeagle and other highly migratory sharks can provide increased certainty in the results since different approaches may provide results of the same magnitude (Cadrin et al., 2014).
Despite the overwhelming evidence of a shared porbeagle stock between Canada and the U.S., fisheries management for the species has varied greatly between the countries over the years. Although Canada has harvested much larger quantities of porbeagle before the closure of their directed fishery in 2013, the U.S. has more consistently allowed low numbers of porbeagle to be harvested. Canada started exploiting porbeagle in the 1990s, provided descriptive stock assessments in the mid-1990s (Hurley, 1995; O’Boyle et al., 1998), and provided an analytical assessment of porbeagle in 1999 (Campana et al., 1999). The 1999 stock assessment produced a catch rate standardization model and yield per recruit analysis using landings, lengths, and tagging data from Norwegian, Canadian, and U.S. vessels in the Northwest Atlantic. The main assumption (supported by the tagging data) was that the Northwest Atlantic porbeagle was one transboundary stock between the U.S. and Canada and international NAFO waters (NAFO areas 2-6; Campana et al., 1999). Canada has since continued to produce assessments of porbeagle to inform Canadian management of the species. All Canadian assessments have assumed that U.S., Canadian, and NAFO porbeagle is one stock (Campana et al., 1999, 2001, 2002a, 2012, 2015). The U.S. has not conducted its own stock assessment for porbeagle, but U.S. management and stock status determinations of the species have relied on the ICCAT/ICES (2009; 2020) stock assessments for Northwest Atlantic porbeagle. Current management strategies and quotas differ between the U.S. and Canada, so increased communication and collaboration between the country’s management organizations (DFO and NMFS) and RMFOs would be beneficial for the success of shared conservation goals. For example, coordinated quotas could reduce recovery time for the current stock, better account for domestic bycatch, and prevent overfishing in the future. Coordinating management of the shared porbeagle stock would be seen as a precautionary approach to international management of a shared transboundary stock. According to ICCAT advice, which the U.S. follows for porbeagle fishery management, “precautionary management measures should be considered for shark stocks where there is the greatest biological vulnerability and conservation concern, and for which there are few data and/or greater uncertainty in assessment results” (ICCAT, 2015). Therefore, broader joint management of the resource should be considered, and the Northwest Atlantic porbeagle should continue to be assessed as one single transboundary stock in U.S., Canadian, and adjacent high seas waters.
The 2009 stock assessment of North Atlantic porbeagle did not include Mediterranean data (ICCAT/ICES, 2009), and the 2020 assessment included Mediterranean catches but set them apart for future consideration (ICCAT, 2020). Based on this comprehensive review of information available on the North Atlantic porbeagle stock structure, excluding the Mediterranean from the Northeast Atlantic porbeagle stock assessment may be a mismatch between biological population structure and management units in the Northeast Atlantic. Although there is little reported catch in the Mediterranean, and they are not expected to have a large influence on stock assessment results, this mismatch could negatively impact fisheries management. The justification given for excluding Mediterranean porbeagle data in the 2020 ICCAT stock assessment for Northeast Atlantic porbeagle was to follow the same format for stock boundaries and assessments as ICCAT does for blue shark (Prionace glauca) and shortfin mako (Isurus oxyrinchus) stock assessments (ICCAT, 2020). However, blue sharks and shortfin mako sharks have different life history characteristics and migration patterns compared to the porbeagle (Kohler et al., 2002; Campana, 2016). As this review has shown, there is no biological or ecological justification to consider the Mediterranean porbeagle a separate stock from the Northeast Atlantic. Accurate stock identification can help reduce uncertainty in the stock assessment as more data may become available (i.e., Mediterranean data) for the data-limited Northeast Atlantic porbeagle. Therefore, future stock assessments should consider the inclusion of catch and effort data on Mediterranean porbeagle from ICCAT and GFCM in the assessment of Northeast Atlantic porbeagle. Re-defining current management units to better reflect biological populations in the Northeast Atlantic would benefit porbeagle stock assessments and potentially reduce data collection requirements for each unit.
Given the current conservation and management situation for North Atlantic porbeagle (i.e., very low commercial landings, CITES-listed), data availability is a limiting factor for future stock assessments and conservation assessments. Stock assessments rely on commercial fishery catch per unit effort (CPUE) time-series data when available and, in data-limited situations, age or length-frequency data to assess reference points, if the stock is overfished, and make predictions about levels of fishing mortality the stock can sustain in the future (Bowlby and Cortes, 2020; ICCAT, 2020). With few commercial porbeagle landings and inconsistent reporting of discards, obtaining representative CPUE series may be challenging, however, new assessment models have been explored (Bowlby and Cortes, 2020; Cortes et al., 2020). Additionally, data collection from bycatch in fisheries not traditionally included in porbeagle stock assessments (i.e., coastal bottom trawls and gillnets) and CPUE from recreationally caught porbeagle may help mitigate some of the data loss from current management and conservation regulations. With little data available, correct stock structure becomes more important as it can reduce data requirements in some situations (i.e., Mediterranean as discussed above). These, and other, research recommendations were included in ICCAT’s 2020 porbeagle assessment.
RFMOs provide the most appropriate processes for international stock assessments of highly migratory species like the porbeagle as they have the ability to unify countries and organizations to create a holistic overview of each species’ biology, ecology, and data needs. While ICCAT, an RFMO focusing on Atlantic tuna fisheries, has been the main arena for highly migratory species stock assessments, other RFMOs such as NAFO, NEAFC, and GFCM could take a more prominent role to improve stock assessments of highly migratory species. For example, in the Northwest Atlantic, this would entail inviting the USA (NMFS), Canada (DFO), and the high seas fisheries (ICCAT) to the table for porbeagle stock assessments hosted by NAFO. In the Northeast Atlantic, a combined host of NEAFC and GFCM, inviting both ICES and ICCAT to contribute, would allow for a more complete assessment of the Northeast Atlantic porbeagle. Increased collaboration between these organizations would be beneficial for data collection, data inclusiveness (i.e., non-tuna and non-pelagic fisheries), the robustness of assessments, and clarity for fishery managers, scientists, and the general public on porbeagle stocks and status.
The management structure of highly migratory species in the North Atlantic has several organizations (i.e., NEAFC, NAFO, GFCM, E.U., ICES, NMFS, DFO, and ICCAT) working on advice, fisheries management, and conservation, and their different jurisdictions may not represent the biological populations of each highly migratory species. The need for an improved international management strategy has been suggested in previous studies (Campana, 2016; Cameron et al., 2019). This review supports the conclusion of previous findings that increased collaboration between the fishery management organizations in the North Atlantic can contribute to improved management, assessments, and conservation of data-limited highly migratory species. Additionally, we provide clear evidence for the most likely stock structure of porbeagle in the North Atlantic and recommend international stock assessments of highly migratory species like the porbeagle to be hosted by overarching RFMOs. No single source of data can provide enough insight for a highly migratory and data-limited species, so interdisciplinary and interagency approaches are particularly well-suited for improving stock identification. Until more research is devoted to interdisciplinary stock identification of porbeagle, this review may serve as a starting point to achieve consistency in the number of stocks, biological population boundaries, and management units amongst organizations working with management, assessments, and conservation.
Acknowledgments
Thank you to Thomas Heimann, Alex Hansell, and Pingguo He, who provided valuable comments on earlier versions of this manuscript. This research was funded by the Andrew E. and G. Norman Wigeland Fund and the Stolt-Nielsen Fund via the American Scandinavian Foundation and the University of Massachusetts, Dartmouth. We would also like to thank the scientists in ICES, WGEF, and WKELASMO that provided valuable information and the reviewers and editor for their constructive feedback and guidance in improving the quality of the final manuscript.
References
Aasen, O. 1963. Length and growth of the Porbeagle (Lamna nasus, Bonnaterre) in the North West Atlantic. Fiskeridirektoratets Havforskningsinstitutt 20–37.
Abaunza, P., Murta, A. G., Campbell, N., et al.2008. Considerations on sampling strategies for an holistic approach to stock identification: The example of the HOMSIR project. Fisheries Research, 89: 104–113. https://doi.org/10.1016/j.fishres.2007.09.020
Anderson, B., Bowlby, H., Natanson, L., et al. 2021.Preliminary estimate of post-release survival of immature porbeagles caught with rod-and-reel in the Northwest Atlantic Ocean. Mar. Ecol. Prog. Ser. 660: 153–159. https://doi.org/10.3354/meps13603
Antoniou, A., and Magoulas, A. 2014. Application of mitochondrial DNA in stock identification. In: S.X. Cadrin, L.A. Kerr, and S. Mariani (Eds.), Stock Identification Methods: Applications in Fisheries Science, 2nd ed., p. 257–285. Elsevier Academic Press, London. https://doi.org/10.1016/B978-0-12-397003-9.00013-8
Begg, G. A. 2004. Life history parameters. In: S.X. Cadrin, K.D. Friedland, and J.R. Waldman (Eds.), Stock Identification Methods: Applications in Fishery Science, 1st ed.. Elsevier Academic Press, London, p. 119–150. https://doi.org/10.1016/B978-012154351-8/50007-1
Bendall, Victoria, J., Ellis, Jim R., Hetherington, Stuart J., et al. 2013. Preliminary observations on the biology and movements of porbeagle (Lamna nasus) around the British Isles. Collect. Vol. Sci. Pap. ICCAT, 69: 1702–1722
Biais, G., Coupeau, Y., Séret, B., et al. 2017. Return migration patterns of porbeagle shark (Lamna nasus) in the Northeast Atlantic: implications for stock range and structure. ICES Journal of Marine Science, 74: 1268–1276. https://doi.org/10.1093/icesjms/fsw233
Bigelow, H. B., and Schroeder, W. C. 1948. Fishes of the Western North Atlantic. Part 1. Lancelets, cyclostomes, sharks. Mem. Sears Found. Marine Research. https://doi.org/10.2307/j.ctvbcd08p.6
Bowlby, H. D., and Cortes, E. 2020. An incidental catch model for porbeagle assessment and status evaluation
Cadrin, S. X. 2010. Interdisciplinary Analysis of Yellowtail Flounder Stock Structure off New England. Reviews in Fisheries Science, 18: 281–299. https://doi.org/10.1080/10641262.2010.506251
Cadrin, S. X. 2020. Defining spatial structure for fishery stock assessment. Fisheries Research, 221: 105397. https://doi.org/10.1016/j.fishres.2019.105397
Cadrin, S. X., Bernreuther, M., Danielsdottir, A. K., et al. 2010. Population structure of beaked redfish, Sebastes mentella: evidence of divergence associated with different habitats. ICES Journal of Marine Science, 67: 1617–1630. https://doi.org/10.1093/icesjms/fsq046
Cadrin, S. X., Kerr, L. A., Mariani, S. 2014. Stock Identification Methods: An Overview. In S.X. Cadrin, L.A. Kerr, and S. Mariani.(Eds.), Stock Identification Methods: Applications in Fisheries Science. 2nd ed. p. 1–5 Elsevier Academic Press, London. https://doi.org/10.1016/B978-0-12-397003-9.00001-1
Cameron, L. W. J., Roche, W., Green, P., et al. 2018. Transatlantic movement in porbeagle sharks, Lamna nasus. Fisheries Research, 207: 25–27. https://doi.org/10.1016/j.fishres.2018.05.014
Cameron, L. W. J., Roche, W. K., Houghton, J. D. R., and Mensink, P. J. 2019. Population structure and spatial distribution of porbeagles (Lamna nasus) in Irish waters. ICES Journal of Marine Science. https://doi.org/10.1093/icesjms/fsz046
Campana, S. E. 2016. Transboundary movements, unmonitored fishing mortality, and ineffective international fisheries management pose risks for pelagic sharks in the Northwest Atlantic. Can. J. Fish. Aquat. Sci., 73: 1599–1607. https://doi.org/10.1139/cjfas-2015-0502
Campana, S. E., Fowler, M., Houlihan, D., et al. 2015. Recovery Potential Assessment for Porbeagle (Lamna nasus) in Atlantic Canada
Campana, S. E., and Gibson, J. 2008. Catch and Stock Status of Porbeagle Shark (Lamna nasus) in the Northwest Atlantic to 2007. NAFO SCR Doc. 08/36. Serial No. N5537
Campana, S. E, Gibson, J., Fowler, M., et al. 2012. Population dynamics of Northwest Atlantic porbeagle (Lamna nasus), with an assessment of status and projections for recovery. Canadian Science Advisory Secretariat Research Document, 2012/096
Campana, S. E., Joyce, W., and Fowler, M. 2010. Subtropical pupping ground for a cold-water shark. Can. J. Fish. Aquat. Sci., 67: 769–773. https://doi.org/10.1139/F10-020
Campana, S. E., Joyce, W., Marks, L., et al. 2002a Population Dynamics of the Porbeagle in the Northwest Atlantic Ocean. North American Journal of Fisheries Management, 22: 106–121. https://doi.org/10.1577/1548-8675(2002)022%3C0106:PDOTPI%3E2.0.CO;2
Campana, S. E., Joyce, W., Marks, L., Harley, S. 2001. Analytical assessment of the porbeagle shark (Lamna nasus) population in the northwest Atlantic, with estimates of long-term sustainable yield. Canadian Stock Assessment Research Document, 2001/067.
Campana, S. E., Marks, L, Joyce W, et al. 1999. An analytical assessment of the porbeagle shark (Lamna nasus) population in the northwest Atlantic. Canadian Stock Assessment Research Document, 1999/158.
Campana, S. E. , Natanson, L. J., Myklevoll, S. 2002b. Bomb dating and age determination of large pelagic sharks. Can. J. Fish. Aquat. Sci., 59: 450–455. https://doi.org/10.1139/f02-027
Cassoff, R. M., Campana, S. E., and Myklevoll, S. 2007. Changes in baseline growth and maturation parameters of Northwest Atlantic porbeagle following heavy exploitation. Can. J. Fish. Aquat. Sci., 64: 19–29. https://doi.org/10.1139/f06-167
CITES, 2013. Proposal for inclusion of Lamna nasus (Bonnaterre, 1788) in Appendix II in accordance with Article II 2(a).
Compagno, L. J. 2001. Sharks of the world. An annotated and illustrated catalogue of shark species known to date. Bullhead, mackeral and carpet sharks (Heterodontiformes, Lamniformes and Orectolobiformes), FAO Species Catalogue for Fishery Purposes No. 1, Vol. 2, 269p.
Cope, J. M., and Punt, A. E. 2011. Reconciling stock assessment and management scales under conditions of spatially varying catch histories. Fisheries Research, 107(1–3), 22–38. https://doi.org/10.1016/j.fishres.2010.10.002
Cortes E, Bowlby, H. D., Carlson, J., et al. 2020. Preliminary sustainability assessment for fishing effects (SAFE) of pelagic longline fisheries on porbeagle sharks and identification of F-based biological reference points
Curtis, T. H., Laporte, S., Cortes, E, DuBeck, G., and McCandless, C. 2016. Status review report: Porbeagle Shark (Lamna nasus). Final Report to National Marine Fisheries Service, Office of Protected Resources. February 2016. 56p.
DeCelles, G. R., and Cadrin, S. X. 2011. An Interdisciplinary Assessment of Winter Flounder (Pseudopleuronectes americanus ) Stock Structure. J. Northw. Atl. Fish. Sci., 43: 103–120. https://doi.org/10.2960/J.v43.m673
DeCelles, G. R., and Zemeckis, D. R. 2014. Acoustic and radio telemetry. In: S.X. Cadrin, L.A. Kerr, and S. Mariani (Eds.), Stock Identification Methods: Applications in Fisheries Science, 2nd ed., p. 397–428. Elsevier Academic Press, London. https://doi.org/10.1016/B978-0-12-397003-9.00017-5
Eagle, T. C., Cadrin, S. X., Caldwell M. E., et al. 2008. Conservation units of managed fish, threatened or endangered species, and marine mammals. NOAA Technical Memorandum NMFS-OPR-37, p. 108
Gauld, J. 1989. Records of porbeagles landed in Scotland, with observations on the biology, distribution and exploitation of the species. Scottish Fisheries Research Report Number 45, p. 16
Goethel, D. R., Kerr, L. A., and Cadrin, S. X. 2016. Incorporating spatial population structure into the assessment–management interface of marine resources. In: C.T.T. Edwards and D.J. Dankel. (Eds.), Management science in fisheries: an introduction to simulation-based methods. New York, p. 319–347
González, M. T., Sepúlveda, F. A., Zárate, P. M., et al. 2020. Regional population genetics and global phylogeography of the endangered highly migratory shark Lamna nasus: Implications for fishery management and conservation. Aquatic Conservation: Marine and Freshwater Ecosystems 31: 3 620–634. https://doi.org/10.1002/aqc.3455
Hammer, C., and Zimmerman, C. 2014. The Role of Stock Identification in Formulating Fishery Management Advice. In: Cadrin, S.X., Friedland, K.D., and Waldman, J. (Eds.), Stock Identification Methods. Elsevier Academic Press, p. 631–658. https://doi.org/10.1016/B978-012154351-8/50031-9
Hanski, I., and Gilpin, M. 1996. Metapopulation Dynamics, Ecology, Genetics, and Evolution. Academic Press, New York
Harrison, A.-L., and Costa, D. P., Winship, A. J., et al. 2018. The political biogeography of migratory marine predators. Nat. Ecol. Evol., 2: 1571–1578. https://doi.org/10.1038/s41559-018-0646-8
Haugen, J. B., and Papastamatiou, Y. 2019. Observation of a porbeagle shark (Lamna nasus) aggregation at a North Sea oil platform. J. Fish. Biol., 95(6) 1496–1499. https://doi.org/10.1111/jfb.14149
Hedrick, P. W. 2000. Genetics of Populations, 2nd ed. Jones and Bartlett, Sudbury, Massachusetts.
Hennache, C., and Jung, A. 2010. Étude de la pêche palangrière de requin-taupe de l’ile d’Yeu. Association pour l’étude et la conservation des sélaciens (APECS).
Heupel, M., Carlson, J., and Simpfendorfer, C. 2007. Shark nursery areas: concepts, definition, characterization and assumptions. Mar. Ecol. Prog. Ser. 337: 287–297. https://doi.org/10.3354/meps337287
Hilborn, R., and Walters, C. 1992. Quantitative Fisheries Stock Assessment: Choice, Dynamics and Uncertainty. Chapman and Hall, New York
ICCAT. 2020. Report of the 2020 porbeagle shark stock assessment meeting. 15–22 June 2020.
ICCAT. 2015. Recommendation by ICCAT on porbeagle caught in association with ICCAT fisheries. Rec 15-06.
ICCAT/ICES. 2009. Report of the 2009 porbeagle stock assessment meeting
ICES. 2022. Benchmark workshop for selected elasmobranch stocks (WKELASMO). February (online) 2022.
Jardim, E., Eero, M., Silva, A., et al. 2018. Testing spatial heterogeneity with stock assessment models. PLoS ONE 13:e0190791. https://doi.org/10.1371/journal.pone.0190791
Jensen, C., Natanson, L. J., Pratt, H., et al. 2002. The reproductive biology of the porbeagle shark (Lamna nasus) in the western North Atlantic Ocean. Fishery Bulletin, 100: 727–738
Kitamura, T., and Matsunaga, H. 2010. Population structure of porbeagle (Lamna nasus) in the Atlantic Ocean as inferred from mitochondrial DNA control region sequences. Collective volume of scientific papers International Commission for the Conservation of Atlantic Tunas, 65: 2082–2087.
Kohler, N, Turner, P. A, Hoey, J. J, et al. 2002. Tag and recapture data for three pelagic shark species, blue shark (Prionace glauca), shortfin mako (Isurus oxyrinchus), and porbeagle (Lamna nasus) in the North Atlantic Ocean. Collective volume of scientific papers International Commission for the Conservation of Atlantic Tunas, 54: 1231–1260.
Kohler, N. E., and Turner, P. A. 2020. Distributions and Movements of Atlantic Shark Species: A 52-Year Retrospective Atlas of Mark and Recapture Data. Marine Fisheries Review, 81: 1–93. https://doi.org/10.7755/MFR.81.2.1
Lipej, L., Uhan, J., Mavrič, B., and Vujčić-Karlo, S. 2016. A record of porbeagle, Lamna nasus (Bonnaterre, 1788), in the Gulf of Trieste with discussion on its occurrence in the Adriatic Sea. Acta Adriatica, 57: 305–314
Mariani, S., and Bekkevold, D. 2014. The nuclear genome: Neutral and adaptive markers in fisheries. In: S.X. Cadrin, L.A. Kerr, and S. Mariani (Eds.), Stock Identification Methods: Applications in Fisheries Science, 2nd ed., p. 297–320. Elsevier Academic Press, London. https://doi.org/10.1016/B978-0-12-397003-9.00014-X
McBride, R. S. 2014. The continuing role of life history parameters to identify stock structure. In: S.X. Cadrin, L.A. Kerr, and S. Mariani (Eds.), Stock Identification Methods: Applications in Fisheries Science, 2nd ed., p.77–96. Elsevier Academic Press, London. https://doi.org/10.1016/B978-0-12-397003-9.00005-9
Natanson, L. J., Deacy, B. M., Joyce, W., and Sulikowski, J. 2019. Presence of a resting population of female porbeagles (Lamna nasus), indicating a biennial reproductive cycle, in the western North Atlantic Ocean. Fishery Bulletin, 117: 70–77. https://doi.org/10.7755/FB.117.1-2.8
Natanson, L. J., Mello, J., and Campana, S. E. 2002. Validated age and growth of the porbeagle shark (Lamna nasus) in the western North Atlantic Ocean. Collect. Vol. Sci. Pap. ICCAT, 54: 1261–1279
Natanson, L. J., Skomal, G. B., Hoffmann, S. L., et al. 2018. Age and growth of sharks: do vertebral band pairs record age? Mar. Freshwater Res., 69: 1440. https://doi.org/10.1071/MF17279
O’Boyle, R. N., Fowler, G. M., and Hurley, P. C. F., et al. 1998. Update on the status of NAFO SA 3-6 porbeagle shark, Lamna nasus. Canadian Stock Assessment Secretariat Research Document, 98/41.
Pade, N. G., Queiroz, N., Humphries, N. E., et al. 2009. First results from satellite-linked archival tagging of porbeagle shark, Lamna nasus: Area fidelity, wider-scale movements and plasticity in diel depth changes. Journal of Experimental Marine Biology and Ecology, 370: 64–74. https://doi.org/10.1016/j.jembe.2008.12.002
Punt, A. E., Haddon, M., and Tuck, G. N. 2015. Which assessment configurations perform best in the face of spatial heterogeneity in fishing mortality, growth and recruitment? A case study based on pink ling in Australia. Fisheries Research, 168: 85–99. https://doi.org/10.1016/j.fishres.2015.04.002
Rigby, C.L., Barreto, R., and Carlson, J., et al. 2018. Lamna nasus: The IUCN Red List of Threatened Species 2019 Global
Ryman, N., Utter, F., and Laikre, L. 1995. Protection of intraspecific biodiversity of exploited fishes. Rev. Fish. Biol. Fisheries, 5: 417. https://doi.org/10.1007/BF01103814
Saunders, R. A., Royer, F., and Clarke, M. W. 2011. Winter migration and diving behaviour of porbeagle shark, Lamna nasus, in the Northeast Atlantic. ICES Journal of Marine Science, 68: 166–174. https://doi.org/10.1093/icesjms/fsq145
Scacco, U, Consalvo, I., DiMuccio, S., and Tunesi, L. 2012. On the by-catch of two porbeagle sharks Lamna nasus in the central Adriatic Sea. Mar. Biodivers. Rec. 5: e61. https://doi.org/10.1017/S1755267212000127
Semba, Y., Yokawa, K., Matsunaga, H., and Shono, H. 2013. Distribution and trend in abundance of the porbeagle (Lamna nasus) in the southern hemisphere. Mar. Freshwater Res., 64: 518. https://doi.org/10.1071/MF12272
Skomal, G., Marshall, H., Galuardi, B., et al. 2021. Horizontal and vertical movement patterns and habitat use of juvenile Porbeagles (Lamna nasus) in the Western North Atlantic.Front. Mar. Sci., 8: 624158. https://doi.org/10.3389/fmars.2021.624158
Skomal, G. B., Zeeman, S. I., Chisholm, J. H., et al. 2009. Transequatorial Migrations by Basking Sharks in the Western Atlantic Ocean. Current Biology, 19: 1019–1022. https://doi.org/10.1016/j.cub.2009.04.019
Sokal, R. R., Rohlf, F. J. 2012. Biometry: the principles and practice of statistics in biological research. W.H. Freeman and Co. New York.
Soldo, A. 2006. Status of the sharks in the Adriatic. In: N., Basusta, C. Keskin, F. Serena and B. Seret (Eds.), The proceedings of the International Workshop on Mediterranean Cartilaginous Fish with Emphasis on Southern and Eastern Mediterranean, Istanbul, Turkey, p. 128–134
Stevens, J. D., Fowler S.L., Soldo, A, et al. 2006. Lamna nasus (Northwest Atlantic population). In IUCN Red List of Threatened Species
Storai, T., Celona, A., Zuffa M., and De Maddalena, A. 2005. On the occurrence of the porbeagle, Lamna nasus (Bonnaterre, 1788) (Chondrichthyes: Lamnidae), off Italian coasts (northern and central Mediterranean Sea): A historical survey. Annales Ser. Hist. Nat., 15: 195–202.
Testerman, C. B. 2014. Molecular Ecology of Globally Distributed Sharks. Doctoral dissertation.
Waples, R. S. 1995. Evolutionarily significant units and the conservation of biological diversity under the Endangered Species Act. In: Nielsen J.L. (Ed.), Evolution and the aquatic ecosystem: defining unique units in population conservation, American Fisheries Society, Bethesda, MD., p. 8–27.
Waples, R. S. 1998. Separating the wheat from the chaff: patterns of genetic differentiation in high gene flow species. Journal of Heredity, 89: 438–450. https://doi.org/10.1093/jhered/89.5.438
Waples, R. S., and Gaggiotti O. 2006. What is a population? An empirical evaluation of some genetic methods for identifying the number of gene pools and their degree of connectivity: Molecular Ecology, 15: 1419–1439. https://doi.org/10.1111/j.1365-294X.2006.02890.x
Zemeckis, D. R., Martins, D., Kerr L. A., and Cadrin S. X. 2014. Stock identification of Atlantic cod (Gadus morhua) in U.S. waters: an interdisciplinary approach. ICES Journal of Marine Science, 71: 1490–1506. https://doi.org/10.1093/icesjms/fsu032
Citation: Haugen, J.B., Skomal, G.B., Curtis, T.H., and Cadrin, S.X. 2022. Interdisciplinary stock identification of North Atlantic porbeagle (
Lamna nasus).
J. Northw. Atl. Fish. Sci.,
53: 1–18. https://doi.org/10.2960/J.v53.m732