Introduction
In 1910, Lea, who was studying herring at the time, showed that scale growth is proportional to body length (Lea, 1910). He found that the relative spacing of annuli was so consistent for single scales that this spacing could be used to back-calculate growth that took place in previous years of the fish’s life. His detailed observations on scale structure and ages were not the first of their kind (Dahl, 1907), and nor were they the last. Havey (1959) reported that scales represent a reliable method for aging Atlantic salmon Salmo salar. Similar observations have been made for a range of species, juvenile steelhead Oncorhynchus mykiss (Beakes et al., 2014) and northern pike Esox lucius (Laine et al., 1991) among them. Although other hard structures, such as otoliths, may be more reliable especially in older age classes of fish (Robillard et al., 1996; Braaten et al., 1999), many state and provincial agencies in the United States and Canada prefer to use scales over these other hard structures for aging common game species (Maceina et al., 2007). Scales require relatively little time and expense to age (Beakes et al., 2014) and, importantly, can be collected non-lethally. This is especially critical when researchers are working with threatened or endangered species.
Atlantic salmon have experienced marked declines across their range, particularly in southern North America, necessitating non-lethal methods of population assessment (Parrish et al., 1998). A recent experimental study by Thomas et al. (2019) found that scale growth and circulus deposition in Atlantic salmon post-smolts was variable and increased with increasing temperatures when food was held constant. They concluded that, while there was a strong relationship between scale and somatic growth, circulus deposition rates must be interpreted in light of the fish’s thermal history in order to be more accurately used as a proxy for growth (Thomas et al., 2019).
The objective of the current study was to describe the scale growth rates and scale circulus deposition rates of marine-stage, net-pen raised Atlantic salmon. Growth and circulus deposition rates were tracked for two sea-winters, and related to time and water temperature, as well as somatic growth. The scale samples used here were collected originally by Sheehan et al. (2005) as part of a larger study to assess phenotypic variation among stocks. We hypothesized that circulus deposition rates would not be constant through time but that they may be related to the thermal experience (water temperature) to which the fish were exposed.
Materials and methods
Field sampling
The field portion of this project was initiated in May 1998 when 6000 1+ Atlantic salmon smolts representing three rivers of origin were stocked into two marine net pen rearing facilities off the coast of Maine. Smolts originated from broodstock that were taken from the Dennys, East Machias, and Machias Rivers. The stocks from these rivers are all part of the Gulf of Maine Distinct Population Segment (GOM DPS), which was listed as Endangered under the Endangered Species Act (Endangered and Threatened Species, 2009) due to continued declines (National Research Council, 2004). The original broodstock were brought into captivity as parr, raised to maturity, and spawned at Craig Brook National Fish Hatchery in East Orland, ME during November 1996. Two thousand smolts from each stock were randomly chosen to be placed in net pens at either Site 1 or 2 (Cross Island or Deep Cove, see Sheehan et al. 2005 for more details [Fig. 1]) on 5 May 1998. The selected smolts were randomly divided between the two Sites, for a total of 3 000 smolts in each net pen (Sheehan et al., 2005). While they were in the net pen the fish were fed to satiation, per industry standards (Sheehan et al., 2005).
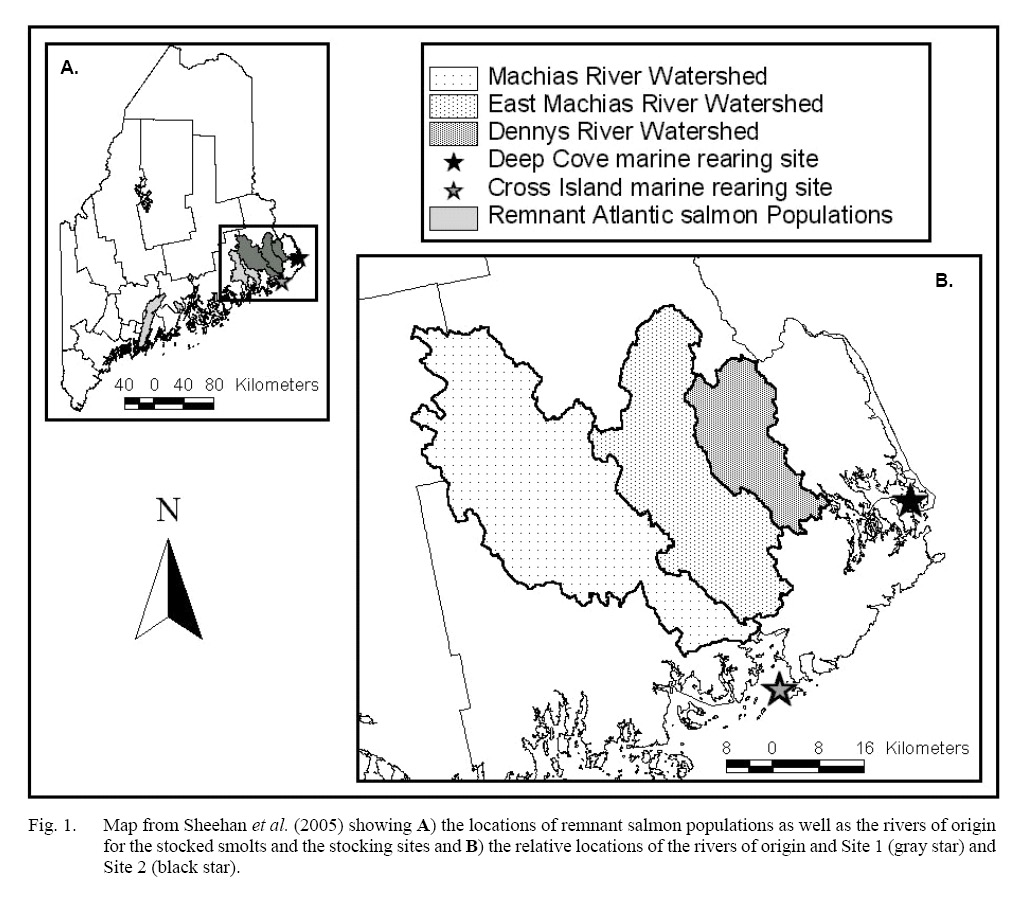
Fig. 1
The salmon were sampled a total of 13 times between May 1998 and June 2000 (Table 1), with the first sampling event (hereafter “Event”) taking place in freshwater rearing facilities prior to release into the net pens and Events 2–13 taking place in seawater. At every Event a seine was pulled through the net pen at each site. At least 30 fish from each stock at each site were measured (mass [grams] and total length [millimeters]) and a sample consisting of 1–16 scales was taken (Sheehan et al., 2005). Fish were sampled only at a single Event and recaptured individuals were released back into the net pen without having a second scale sample taken to avoid collecting regenerated scales from standardized scale sampling areas below the dorsal fin. However, recaptured individuals were weighed and measured each time they were recaptured. Previously sampled fish were identified with a uniquely-coded colored Visual Implant Elastomer tag (VIE, Northwest Marine Technology, Inc.). The colors of these VIE tags were specific to each stock and therefore also useful for stock identification. Sheehan et al. (2005) also obtained hourly water temperatures across the duration of the study at each of the two rearing sites using remote temperature loggers. At the end of the initial study the adults at Site 1 were released into the wild. However, disease concerns at Site 2 necessitated that the fish be sacrificed rather than stocked. The disease in question, infectious salmon anemia (ISA), was detected in the same bay as these fish, and all fish used in the samples reported by this paper were asymptomatic. Because presence of the disease does not necessarily imply infection (McBeath et al., 2009), and because all of the salmon used in this study were asymptomatic for ISA, it is unlikely that this disease influenced growth rates or scale circulus deposition patterns.
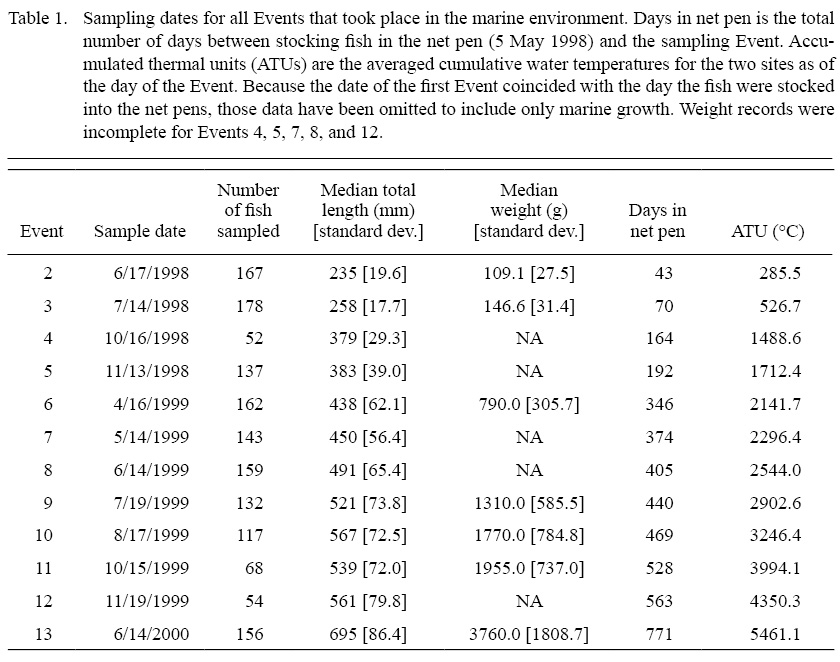
Table 1
Laboratory methods
Scales were air-dried after collection, and cleaned by gently rubbing them between the fingertips in a dish of soapy water. Before and after mounting, the scales were placed in paper scale envelopes and stored in cardboard boxes that were kept indoors. Beginning in the fall of 2017, the slides were photographed under either 2.5x or 10x magnification on a ZEISS Axioplan 2 microscope (ZEISS International, Oberkochen, Germany) with a microscope-mounted digital camera (SPOT Insight 2 MP Color Mosaic; Diagnostic Instruments, Sterling Heights, Michigan). Previous to recording any data from the scales, a photograph of a stage micrometer at both 2.5x and 10x magnification was used to produce an appropriate calibration for the images. Each scale was uniquely coded based on the fish identifier coupled with a sequential numbering on each slide. All scales were photographed regardless of condition or regeneration status, but scales with regenerated centers or cracked edges were not processed further because they may not be useful for accurately determining age or growth (Blair, 1942; McNicol and MacLellan, 2010).
One reader processed each photograph of usable scales, which resulted in 1–11 replicates per fish. The number of replicates equaled the number of usable (whole, non-regenerated) scales available for each fish. We did not use the same number of replicates for each fish because this would have required using only one scale per fish, as some fish only had one usable scale available. Instead, we averaged the scale size and circulus number of all available scales for fish which had multiple usable scales. To obtain these measurements, the reader obtained circuli counts and spacing for each usable scale using ImagePro Premier software (Media Cybernetics, 2012), in which a calibrated line, placed by the reader, was applied to the scale image that measured the total length from the center of the nucleus along the longest axis of the scale. ImagePro automatically placed markers on the line at the outside edge of every circulus based on the light/dark transition in the pixels. These markers could be examined and manually shifted or removed by the reader to make sure they had been placed on actual circuli. For each image, ImagePro also generated a data table that contained the number of markers (circuli) attributed to the scale and the distance from the nucleus to each circulus, as well as the total distance from the nucleus to the outside edge of the scale. The distances from the nucleus to each circulus were retained but are not reported here. The scale length and number of circuli on each scale were averaged among individual fish.
Data analysis
Reading multiple scales from the same individual can reduce sampling error, especially when sample sizes are low (Haraldstad et al., 2016). We measured all of the usable scale available for each fish, which ranged from 1–11 scales with a median of 2 scales per fish. Circulus counts and scale radius measurements among scales collected from the same individual fish were averaged.
Scale radius and fish total length were compared using simple linear regression (SLR). Differences in scale growth rate and circulus deposition rate between net pen sites were compared using a Welch two-sample t-test (W-2s t-test) with α = 0.05 because the variances in growth rates and circulus deposition rates were found to be unequal, and the W-2s t-test should be robust to non-normality. Rates were compared as both daily rates, and relative to water temperature. Accumulated thermal units (ATUs) were used to describe the thermal experience of the fish throughout the study. Accumulated thermal units were obtained by summing the mean daily water temperatures (°C) between Events. All water temperatures above 0°C were included in the calculation of ATU and negative temperatures were treated as 0°C (Boyd et al., 2010; Chezik et al., 2014). Scale growth rates and circulus deposition rates relative to time and ATUs were also compared among stocks of origin using ANOVA with α = 0.05.
Scale growth rates and circulus deposition rates were averaged among sampling Events across the duration of the study and among stocks and sites to ascertain the presence of any relationships between these rates and either time or water temperature. Growth and circulus deposition were calculated between Events, so there are a total of 12 growth/ circulus deposition intervals among the 13 sampling Events. However, data from Event 1 were omitted because Event 1 took place in the freshwater rearing facilities, leaving a total of 11 growth/circulus deposition intervals for the analysis. Using these results we also calculated the number of days required for a single scale circulus to form.
Results
A total of 1 525 fish among all stocks and net pen sites was sampled over the duration of the project. The difference in mean water temperature between Site 1 and Site 2 was only 0.15°C (Site 1=7.45°C, Site 2=7.6°C). Therefore, the temperatures from the two sites were averaged, and the resulting temperature time series was used for all further analyses (Fig. 2). Additionally, two large gaps in temperature data from Site 2 made it impossible to calculate reliable scale growth rates or circulus deposition rates on a pen-specific basis
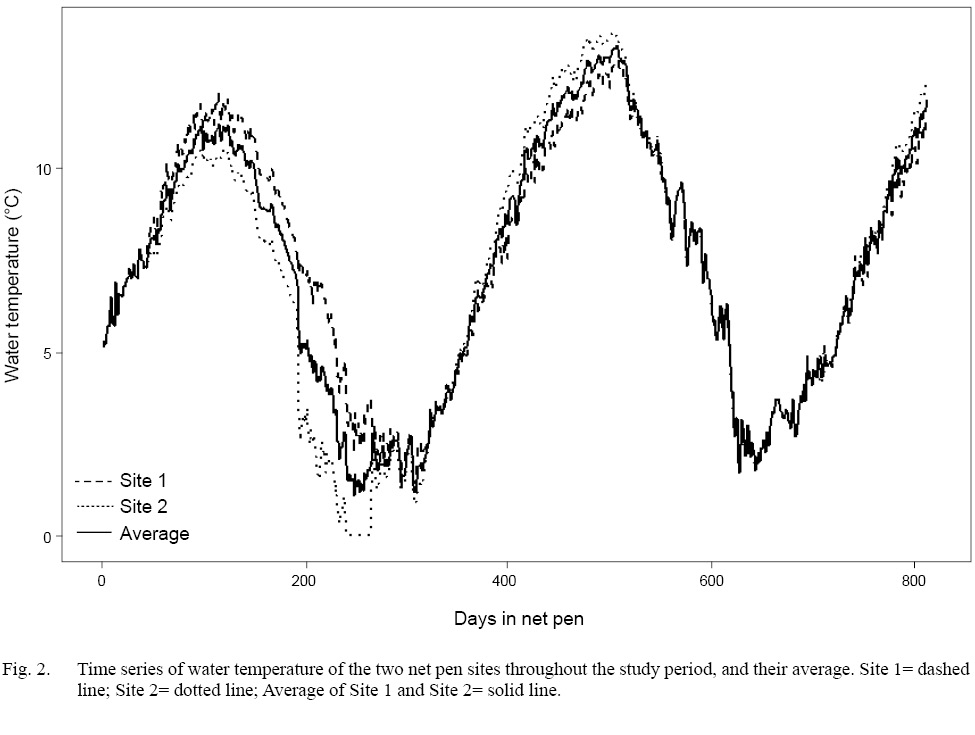
Fig. 2
Daily scale growth rate and daily scale circulus deposition rate were higher at Site 1 than at Site 2 (daily scale growth: W-2s t-test, t=3.6, P<0.05; daily circulus deposition: W-2s t-test, t=2.8, P<0.05). As expected, when scale growth and circulus deposition rate were related to ATUs both rates were higher at Site 1 than at Site 2 (daily scale growth: W-2s t-test, t=3.5, P<0.05; daily circulus deposition: W-2s t-test, t=2.7, P<0.05). There were no differences in scale growth rates or circulus deposition rates among stocks for either daily rates or rates compared to ATUs (daily scale growth: ANOVA F2,1522=0.42, P>0.05; daily circulus deposition: ANOVA F2,1522=0.31, P>0.05; scale growth per ATU: ANOVA F2,1522=0.31, P>0.05; circulus deposition per ATU: ANOVA F2,1522=0.28, P>0.05). Therefore, the data for scale growth rate and circulus deposition rate, respectively, were combined for all stocks within a site but the sites were treated separately for the remainder of the analysis.
Relationship of scale growth rate to days spent in net pen and water temperature
Scale radius and fish total length showed a strong relationship at both sites when the data were considered as a whole (Site 1: SLR, adjusted R2=0.95, P<0.001; Site 2: SLR, adjusted R2=0.93, P<0.001 [Fig. 3]). However, daily growth rates showed a non-linear, negative trend through time (Fig. 4a–b). Among Events, the daily scale growth rate was not consistent (Site 1: ANOVA, F1,692=1207, P<0.05; Site 2: ANOVA, F1,829=1272, P<0.05). The same trend was evident in the relationship between scale growth and water temperature through time (Site 1: ANOVA, F1,692=864.8, P<0.001; Site 2: ANOVA, F1,829=1229, P<0.001 [Fig. 4c–d]).
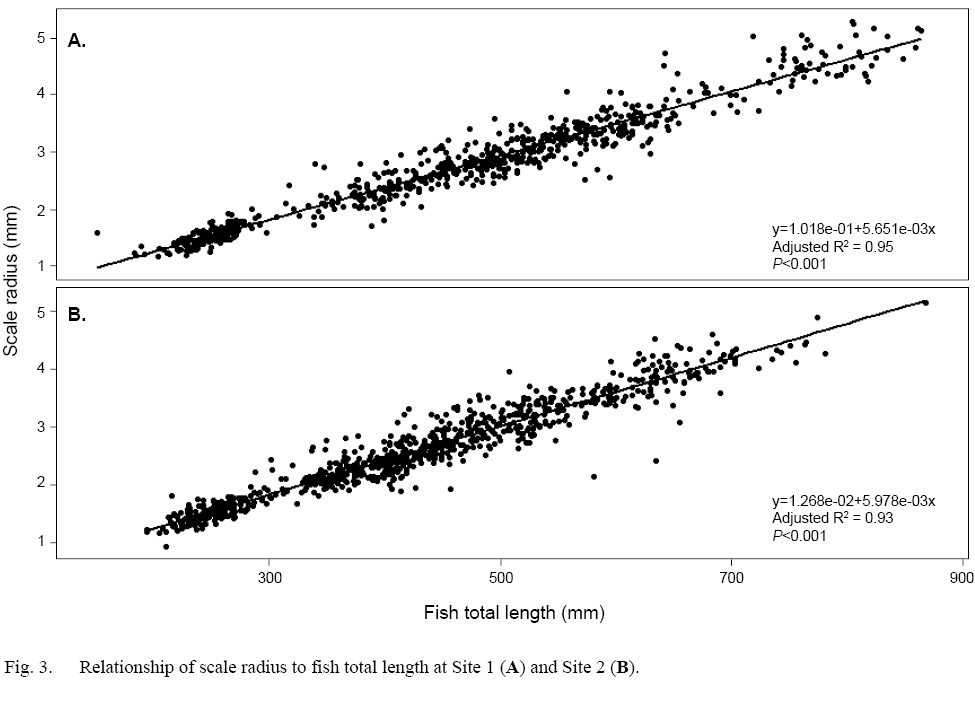
Fig. 3
Relationship of circulus deposition rate to days spent in net pen and water temperature
Circulus deposition rate showed similar patterns to scale growth rate through time. Circulus deposition rate was not constant through time (Site 1: ANOVA, F1,692=1183, P<0.001; Site 2: ANOVA, F1,829=1030, P<0.001) and showed a sharp decrease throughout the first five sampling Events (192 days post-stocking, [Fig. 5a–b]). The relationship between circulus deposition rate and water temperature was also not constant among Events, with the steepest decrease in circulus deposition rates occurring among the first three sampling Events (Site 1: ANOVA, F1,692=898.9, P<0.001; Site 2: ANOVA, F1,829=1036, P<0.001 [Fig. 5c–d]).
When scale circulus deposition rate was measured on a daily interval, each circulus required an average of 2.7 days to form at Site 1, with a range of 0.79-10.4 days. At Site 2, a single circulus formed on average every 3 days, with a range of 0.79–12 days. When considered relative to water temperature, a single circulus was deposited when a fish had experienced 5–75.5 ATU, with a mean of 19 ATU per circulus at Site 1. The temperature experience required for a single circulus to form on fish at Site 2 was similar, with an average of 20.5 ATU and a range of 5.3–82 ATU. The highest circulus deposition rates relative to both time and ATUs occurred between entrance to the marine environment and Event 2, the first marine sampling Event, while the lowest rates occurred among the final Events of the study.
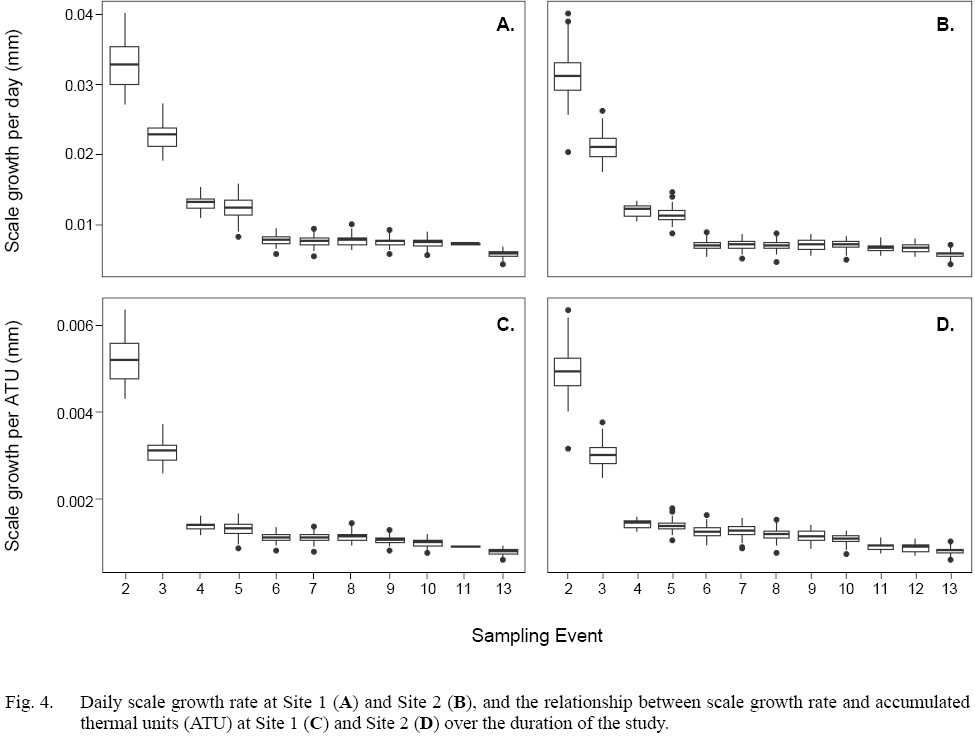
Fig. 4
Discussion
Our study demonstrated that scale growth rates and circulus deposition rates in marine-stage Atlantic salmon are not constant through time. Daily growth and circulus deposition rates decreased over the course of our study, with the highest rates occurring during the first year of marine habitation and the lowest rates occurring when the study was terminated at the end of two and a half growing seasons. The same trends were seen when scale growth rate and circulus deposition rate were plotted relative to thermal experience.
Decreasing somatic growth as fish approach sexual maturity could explain the trends seen in scale growth and circulus deposition rate. At the end of the original study, the salmon were 3+ years old and had spent two winters (1998–1999, 1999–2000) in the sea. This is a typical age for US Atlantic salmon to make their first spawning migration (Gardner, 1976). However, the maturity status of the fish used in this study was not recorded, so it is not known how sexual maturity may have affected scale growth and circulus deposition rates for these particular fish.
Studies of Pacific salmonids have also found that marine growth, as evidenced by scale circulus spacing and circulus deposition rate, decreases through time, and may be at least partially attributable to a reduction in somatic growth as the fish ages. Barber and Walker (1988) found that scale circulus spacing decreased between the first and second year at sea in adult Sockeye salmon O. nerka. Fisher and Pearcy (2005) compared circulus deposition rates in juvenile and maturing Coho salmon O. kisutch. On average, juvenile coho salmon deposited a new scale circulus every 5.3 days, whereas maturing fish deposited a new circulus every 7.6 days. Thomas et al.(2019) reported a rate that ranged between 16.2 days per circulus for Atlantic salmon held at low water temperatures (6°C) to 5.1 days per circulus for fish held at higher temperatures (15°C). They found that circulus deposition rate was also affected by the consistency of food availability. These circulus deposition rates are similar to those seen in our study fish when the first five sampling Events, which cover the first year at sea, are compared with later sampling Events.

Fig. 5
Barber and Walker (1988) also found strong correlations between increasing photoperiod and increasing fish growth. They attributed some of the patterns in circuli spacing that they saw to changes in food availability (Barber and Walker, 1988). Neither the photoperiod nor the food availability experienced by our fish represented natural conditions. Because Atlantic salmon in the wild are transient and spend a majority of their time at high latitudes, they experience a greater seasonal fluctuation in photoperiod than salmon that are confined to the Maine coast. In addition, our fish were fed to satiation, a condition which undoubtedly does not occur in the wild. However, net pen studies such as this one can be useful for conducting long term sampling of fish held under semi-natural conditions.
Fish in the current study were only sampled during a single sampling Event; any fish that were recaptured at subsequent Events were put back in the net pen and a new fish obtained in their place. Future studies aimed at gaining a detailed understanding of Atlantic salmon post-smolt scale growth rates and circulus deposition rates would benefit from frequent, repeated sampling of known individuals. Sampling events outside of the growing season would also yield beneficial information about seasonal changes in growth and circulus deposition rates. Such a sampling scheme could retain important information about individual variability in growth and circulus deposition rates and also allow for a more detailed understanding of scale formation and growth relative to different aspects of the fish’s life history.
The present study expands upon previous work on Atlantic salmon marine-stage growth (i.e., Thomas et al., 2019) by tracking growth and circulus deposition rates in the marine environment through two sea-winters, under a semi-natural temperature and photoperiod regime. Under these conditions, which more closely mimic those experienced by fish in the wild than previous laboratory studies, both scale growth and circulus deposition rates were non-constant and decreased through time. Acknowledgement of these fluctuating growth and circulus deposition rates in further studies of Atlantic salmon could help researchers obtain more detailed information about growth patterns in this species.
Acknowledgments
In-kind support was provided by the U.S. Geological Survey Maine Cooperative Fish and Wildlife Research Unit. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Special thanks to L. Carlson for providing a thorough review of an earlier version of the manuscript, to C. Dillingham and Z. Hamel for photographing scales, and to K. Job and L. Izzo for technical assistance. No animals were used for this study.
References
Barber, W. E., and Walker, R. J. 1988. Circuli spacing and annulus formation: is there more than meets the eye? The case for sockeye salmon, Oncorhynchus nerka. Journal of Fish Biology, 32: 237–245. https://doi.org/10.1111/j.1095-8649.1988.tb05357.x
Beakes, M. P., Sharron, S., Charish, R., Moore, J. W., Satterthwaite, W. H., Sturm, E., Wells, B. K., Sogard, S. M., and Mangel, M. 2014. Using scale characteristics and water temperature to reconstruct growth rates of juvenile steelhead Oncorhynchus mykiss. Journal of Fish Biology, 84: 58–72. https://doi.org/10.1111/jfb.12254
Blair, A. A. 1942. Regeneration of the scales of Atlantic salmon. Journal of the Fisheries Research Board of Canada, 5c: 440–447. https://doi.org/10.1139/f40-045
Boyd, J. W., Oldenburg, E. W., and McMichael, G. A. 2010. Color photographic index of fall Chinook salmon embryonic development and accumulated thermal units. PLoS ONE, 5(7): e11877. https://doi.org/10.1371/journal.pone.0011877
Braaten, P. J., Doeringsfeld, M. R., and Guy, C. S. 1999. Comparison of age and growth estimates for river carpsuckers using scales and dorsal fin ray sections. North American Journal of Fisheries Management, 19: 786–792. https://doi.org/10.1577/1548-8675(1999)019<0786:COAAGE>2.0.CO;2
Chezik, K. A., Lester, N. P., and Venturelli, P. A. 2014. Fish growth and degree-days I: selecting a base temperature for a within-population study. Canadian Journal of Fisheries and Aquatic Sciences, 71: 47–55. https://doi.org/10.1139/cjfas-2013-0295
Dahl, K. 1907. The scales of the herring as a means of determining age, growth, and migration. Report on Norwegian Fishery and Marine-Investigations Vol. II 1907 No. 6. 39p.
Endangered and Threatened Species; Designation of Critical Habitat for Atlantic Salmon (Salmo salar) Gulf of Maine Distinct Population Segment, 74 Fed. Reg. 29299 (June 19, 2009).
Fisher, J. P., and Pearcy, W. G. 2005. Seasonal changes in growth of coho salmon (Oncorhynchus kisutch) off Oregon and Washington and concurrent changes in the spacing of scale circuli. Fishery Bulletin, 103: 34–51. https://ir.library.oregonstate.edu/concern/articles/gq67js639
Gardner, M. L. G. 1976. A review of factors which may influence the sea-age and maturation of Atlantic salmon Salmo salar L. Journal of Fish Biology, 9: 289–327. https://doi.org/10.1111/j.1095-8649.1976.tb04680.x
Haraldstad, T., Haugen, T. O., Borgstrom, R., and Jonsson, B. 2016. Increased precision of growth data gained by reading multiple scales from each individual of Atlantic salmon (Salmo salar). Fauna norvegica, 36:1–7. https://doi.org/10.5324/fn.v36i0.1954
Havey, K. A. 1959. Validity of the scale method for aging hatchery-reared Atlantic salmon. Transactions of the American Fisheries Society, 88: 193–196. https://doi.org/10.1577/1548-8659(1959)88[193:VOTSMF]2.0.CO;2
Laine, A. O., Momot, W. T., and Ryan, P. A. 1991. Accuracy of using scales and cleithra for aging northern pike from an oligotrophic Ontario lake. North American Journal of Fisheries Management, 11: 220–225. https://doi.org/10.1577/1548-8675(1991)011<0220:AOUSAC>2.3.CO;2
Lea, E. 1910. Contributions to the methodics in herring-investigations. ICES Journal of Marine Science, 1: 7–33. https://doi.org/10.1093/icesjms/s1.53.7
Maceina, M. J., Boxrucker, J., Buckmeier, D. L., Gangl, R. S., Lucchesi, D. O., Isermann, D. A., Jackson, J. R., and Martinez, P. J. 2007. Current status and review of freshwater fish aging procedures used by state and provincial fisheries agencies with recommendations for future directions. Fisheries, 32: 329–340. https://doi.org/10.1577/1548-8446(2007)32[329:CSAROF]2.0.CO;2
McBeath, A. J. A., Bain, N., and Snow, M. 2009. Surveillance for infectious salmon anaemia virus HPR0 in marine Atlantic salmon farms across Scotland. Diseases of Aquatic Organisms, 87: 161–169. https://doi.org/10.3354/dao02128
McNicol, R. E., and MacLellan, S. E. 2010. Accuracy of using scales to age mixed-stock Chinook salmon of hatchery origin. Transactions of the American Fisheries Society, 1398: 727–734. https://doi.org/10.1577/T09-033.1
National Research Council of the National Academies. 2004. Atlantic salmon in Maine. The National Academies Press, Washington, DC.
Parrish, D. L., Behnke, R. J., Gephard, S. R., McCormick, S. D., and Reeves, G. H. 1998. Why aren’t there more Atlantic salmon (Salmo salar)? Canadian Journal of Fisheries and Aquatic Sciences, 55: 281–287. https://doi.org/10.1139/d98-012
Robillard, S. R., and Marsden, J. E. 1996. Comparison of otolith and scale ages for yellow perch from Lake Michigan. Journal of Great Lakes Research, 22: 429–435. https://doi.org/10.1016/S0380-1330(96)70967-X
Sheehan, T. F., Kocik, J. F., Cadrin, S. X., Legault, C. M., Atkinson, E., and Bengtson, D. 2005. Marine growth and morphometrics for three populations of Atlantic salmon from eastern Maine, USA. Transactions of the American Fisheries Society, 134: 775–788. https://doi.org/10.1577/T04-067.1
Thomas, K., Hansen, T., Brophy, D., Maoileidigh, N. O., and Fjelldal, P. G. 2019. Experimental investigation of the effects of temperature and feeding regimes on scale growth in Atlantic salmon Salmo salar post-smolts. Journal of Fish Biology, 94: 896–908. https://doi.org/10.1111/jfb.13971
: Peterson, E., Sheehan, T.F., and Zydlewski, J.D. 2021. Scale growth rates and scale circulus deposition rates of marine-stage Atlantic salmon
raised under semi-natural conditions. J. Northw. Atl. Fish. Sci., 52: 19–27.