J. Northw. Atl. Fish. Sci., Vol. 51: 105–116
Publication (Upload) date: 13 Nov 2020
Gabriel Canani
Programa de Pós-graduação em Oceanografia Biológica, Instituto de Oceanografia, Universidade Federal de Rio Grande,
Avenida Itália, Km 8, Campus Carreiros,
Rio Grande, RS, Brasil, CEP 96203-900
Maria Cristina Oddone*
Instituto de Ciências Biológicas, Setor de Morfologia, Universidade Federal de Rio Grande, Avenida Itália, Km 8, Campus Carreiros,
Rio Grande, RS, Brasil, CEP 96203-900
*mcoddone@gmail.com
Canani, G. and Oddone, M.C. 2020. Reproductive biology of Isurus oxyrinchus captured by the south Brazilian surface longline commercial fleet in the Southwest Atlantic Ocean, with data on CPUE and size distribution by sex. J. Northw. Atl. Fish. Sci., 51: 105–116. https://doi.org/10.2960/J.v51.m724
Abstract
Knowledge of reproductive parameters is necessary to efficiently evaluate and manage fishing stocks. Shortfin Mako constitutes the second most captured shark by the longline hook fleet situated in Rio Grande, RS, Brazil. However, little is known about the reproductive traits or abundance of this species in the Southwest Atlantic. Here we report on size and maturity, from 37 males and 46 females sampled in scientific cruises between September 1996 and August 1999, and commercial fishing cruises between December 2014 and September 2016, in South Brazilian Waters and Northern Uruguayan Waters. First results for male maturity are presented. Males between 119.0 and 270.0 cm TL (Total length) including all maturity stages were captured; eighteen adults, nine juveniles and ten immature individuals. The observed size at maturity was between 137 and 182.0 cm TL. Maturity ogive analyses indicate an L50 of 180.1 cm TL and L90 was 199.0 cm TL. Juveniles presented testicle weight between 84.4 and 92.2 g, while adult weights were between 158.4 and 352.8 g. Only immature females were captured (n = 46), with sizes between 104.0 and 230.0 cm TL, and oviduct width between 4.1 and 7.15 mm. The CPUE varied between 0.57 and 2.38 individuals per thousand hooks. Overall sex ratio slightly favored females, 1.24:1 (F|M).
Key words: South Brazil, reproductive biology, population structure, pelagic shark
PDF
Download Citation Data

Citation to clipboard
 Reference management software (Endnote, Mendeley, RefWords, Zotero & most other reference management software)
Reference management software (Endnote, Mendeley, RefWords, Zotero & most other reference management software)
LaTex, BibDesk & other specific software
Introduction
Reproductive parameters such as size or age at maturity are important for evaluating recruitment of sharks, and therefore evaluating fisheries and population status (Holden, 1974; Cortés et al. , 2012). Acquiring information on sexual maturity can inform biological and ecological traits of the species. These traits may be even sex biased, given that sexual dimorphism and site segregation are common in sharks (Francis and Duffy 2005; Semba et al. eet al. t al., 2011, Tsai et al. , 2014). Tsai et al. (2014) indicated from a fishery management perspective that ignoring the differences in population growth rates between sexes, as well as in social structure et al. i.e., unsexed stock management can underestimate population decline risks.
The family Lamnidae (“mackerel” sharks, order Lamniformes), includes the genera et al. Carcharodon Smith, 1838, et al. Lamna Cuvier, 1816, and et al. Isurus Rafinesque 1810 (Compagno, 2005). In regard to the Shortfin Mako et al. Isurus oxyrinchusRafinesque, 1810, Shortfin Mako is endothermic, being able to maintain higher t emperatures than the surrounding water with counter-current vascular heat exchange (Carey and Teal, 1969). This physiological trait facilitates a high movement and migratory capacity. The species is oceanic, semipelagic and littoral and distribution is circumglobal in temperate and tropical seas. In the Western Atlantic, Shortfin Mako occurs from Newfoundland (Canada) (Casey and Kohler, 1992) to northern Argentina (Compagno, 1984; 1990).
In the South and Central Atlantic waters, Shortfin Mako is the second most captured shark in longline fisheries (Barreto et al., 2016a). Having such a wide range exposes the Shortfin Mako to a large number of fishing fleets, the longline hook fishery being the primary threat (Cortés et al. , 2010, Barreto et al. , 2016a). Barreto et al. , (2016a) reviewed historical catches by the longline fishery fleets in the South Atlantic Ocean, identifying three main phases. The first (1979–1997), a period with low effort employing multifilament lines targeting tunas was characterized by increasing shark catches as the fishery developed. The second phase (1998–2007) was a period of expansion and monofilament line was introduced targeting sharks as well as tunas, resulting in a decline of 55% in Shortfin Mako catches. Finally, during a third phase, shark catches stabilized at a lower level. Mourato et al. , (2011) found high percentages of captures of the Shortfin Mako, regardless of the targeted species in longline fisheries off the southeastern coast of Brazil. Shortfin Mako represent 2.6% of total catches in fisheries targeting blue sharks, et al. Prionace glauca (Linnaeus 1758), 3.0% in fisheries for swordfish et al. Xiphias gladius Linnaeus, 1758 and 4.4% in multi species fisheries (Mourato et al. , 2011). The overall declining trend of the Shortfin Mako captures could suggest a depletion of the stock, and thus an endangered status to the species (Barreto et al. , 2016a), currently categorized by the IUCN as “Endangered” both globally and regionally, in the Atlantic (Rigby et al. , 2018).
With respect to reproduction strategies, oophagy was documented in all members of the Lamniformes, including the Shortfin Mako (Gilmore, 1993; Mollet et al. et alet al. ., 2000; Joung and Hsu, 2005). Following initial yolk-sac nutrition, oophagy, a type of matrotrophic viviparity occurs where intrauterine embryos ingest unfertilized eggs continuously produced by the mother (Hamlett and Koob, 1999). As the embryos consume and store the yolk present in the ova, they develop an expanded abdomen called “cardiac” or “yolk” stomach, characteristic and exclusive of the Lamniformes (Gilmore 1993, 2005; Mollet et al. , 2000; Wyffels, 2009). Approximately two months before birth, the embryos consume the yolk and develop livers proportionally identical to the adults in relation to their weight (Gilmore, 1993, 2005; Mollet et al. 2000; Joung and Hsu, 2005). Among oophagic species, the adelphophagy, embryophagy or “intrauterine cannibalism” has been recorded in et al. I. oxyrinchus and et al. Carcharias taurus Rafinesque, 1810 (Mollet et al. , 2000; Gilmore, 2005; Joung and Hsu, 2005).
Shortfin Mako size at sexual maturity ranges between 156 and 210 cm for males and between 256 and 285 cm for females, with great variation among regions and studies (Mollet et al. , 2000; Joung and Hsu 2005; Semba et al., 2011, see Table 3 for details). Regardless of the growing amount of information on biology and reproductive parameters of the Shortfin Mako in the last 30 years, there is still a considerable lack of information about this species, as for most of Lamniformes (Gilmore 1993, 2005; Mollet et al. , 2000). Moreover, size at maturity estimates for male Shortfin Mako were not available for the South West Atlantic.
Stevens (2008) stated that, in spite of being commonly captured, the biology of this species is still not well understood. The economical and ecological importance of the species, the apparent declining populations trend, lack of reproductive studies in the Southwest Atlantic, discrepancies among studies and differences among populations of the Shortfin Mako highlight the need to acquire fishing and reproductive biology information for this species. The aim of this study was to study the reproductive biology of et al. Isurus oxyrinchus captured by the south Brazilian surface longline commercial fleet in the Southwest Atlantic Ocean, and to provide data on CPUE and size distribution by sex. Here, we present population structure data, average size per season sampled and reproductive parameters.
Material and methods
Study Area
The fishing cruises were carried out in the area situated in the Southwest Atlantic Ocean, between latitude 29° and 36° S, and longitude 47° and 53° W (Fig. 1), in depths between 140 and 2200 m. This area corresponds to the southernmost Brazilian states; Santa Catarina and Rio Grande do Sul, respectively and including the northernmost Uruguayan waters. The main currents acting on the surface waters are the Brazil Current, which flows southward, characterized by warm and oligotrophic waters, and the Malvinas (Falklands) Current, flowing northward, composed by Antarctic Circumpolar Waters which are mixed with coastal waters coming from the La Plata River, these mixed waters are characterized by cold and nutrient-rich waters. These two currents with opposite flows meet each other, forming the South Atlantic Subtropical Convergence (Garcia, 1997).
Sample collection
Samples were collected during six trips aboard a 22 m length steel longliner, fishing in the south Brazilian inner and outer shelves from December 2014 to September 2016 (Table 1). An additional specimen was sampled during a single fish landing event in the port of the city of Rio Grande. Hooks were fixed using a steel line, appropriate for capturing large sharks and swordfishes. The usual set consisted of 800 hooks, varying between 600 and 1050 hooks. Also, data from scientific longline cruises obtained in the same area between 1996 and 1999 by the project ARGOS (conducted by the former Laboratório de Elasmobrânquios e Aves Marinhas, Instituto de Oceanografia, Universidade Federal do Rio Grande) were also included (Fig.1). Catch per unit effort (CPUE) was calculated only for fisheries cruises, using 800 hooks per set for effort calculation. The total effort per cruise was not available for the scientific cruises.

Fig. 1
Biological and biometric sampling
The external measurements were taken at sea, as the fish were brought on board. For each specimen, total length (TL), fork length (FL) and interdorsal space (IS) were recorded to the nearest cm below. For males, the postcloacal length of the clasper (CL) was measured from the clasper distal end to the posterior margin of the cloaca opening, according to Maia et al.(2007). Clasper calcification was recorded manually, with the claspers being classified as ‘rigid’ or ‘flexible’ for maturity assessment. Due to access difficulties in biological sampling for reproduction assessment intrinsic of this species (also reported by Maia et al. , 2007, and Barreto et al. , 2016b), the testicles of seven specimens were weighed. Testicle weight (g) (including the epigonal organ) was recorded using an electric scale (0.1 g precision) considering both testicles together. Also, uterus width was recorded for 12 females, being measured in the middle region of the oviduct.
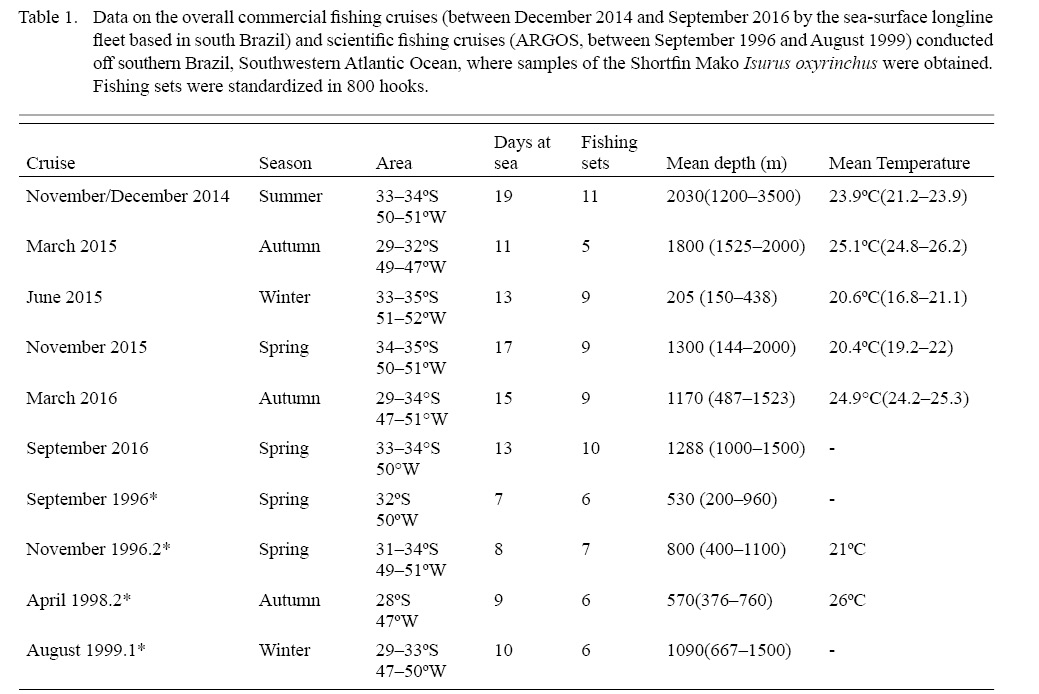
Table 1
Analysis and statistics
To assess sexual maturity, criteria proposed by Semba et al. (2011) for the shortfin mako shark were applied. To determine maturity in males, the calcification and mobility of the clasper were examined, and maturity was as follows: stage 1-immature (not calcified claspers); stage 2-juvenile (semi-rigid and calcified claspers with low mobility (clasper rotation forward along the caudal-cephalic body axis) and no spurs) and stage 3-adult (calcified, high mobility, spurs).
The normality of TL and CL measurements was tested using the Shapiro-Wilk test. Morphometrics were analyzed by plotting all measurements using linear regression (Pratt and Casey, 1983), to observe the relationship between total length and clasper length. The sex ratio was established as the ratio of total females to total males. A statistical X² test was applied to test differences between sexes. The logistic model Y = [1 + exp{−(a + bX}] −1 (sensu Mollet et al. , 2000) was fitted to the relationship between the percentage of mature males (Y) and TL classes of 10 cm (X), and et al. a and et al. b are parameters estimated by a generalized linear model (GLM) with a binomial distribution. Statistical analyses and figures were performed using the program R, ver. 3.2.2 (R Development Core Team, 2019).
Ethical considerations
For the collection of specimens of et al. I. oxyrinchus on board the fishing vessels, permission of the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) and Sistema de Autorização e Informação em Biodiversidade (SISBIO) was requested, license number 45279-1. This research is part of the research project “The biology and conservation of shark populations in the extreme south of Brazil” registered at the Universidade Federal do Rio Grande under process number 814440/2014, with awareness of the Ethics Committee in Animal Use (Comissão de Ética Em Uso Animal – CEUA of the same university. No experimental work was undertaken with the specimens collected.
Results
Reproductive biology
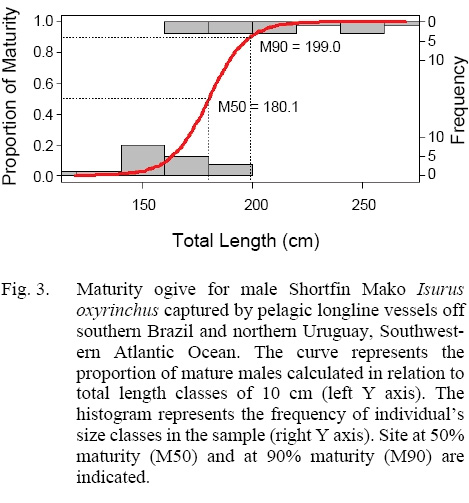
The sample analyzed (Table 2) was composed of immature males (stage 1, et al. n = 10), with (CL) ranging from 6.0 to 12.3 cm juveniles (stage 2, et al. n = 9), with (CL) from 9.5 to 22 cm and adults (stage 3, et al. n = 18) showing a CL range between 18.5 and 35.4 cm. The development of the clasper correlated with TL, exhibited three distinct phases. The first, with slow clasper growth and samples between 119.0 and 162.0 cm TL, followed by a rapid growth between 137.0 and 182.0 cm TL, and when maturity is reached, showing a slow growth once again (Fig. 2). The observed size (TL) at first maturity in the sample corresponded to the range 168.0–182.0 cm TL (TL of the smallest mature individual and largest immature individual, respectively). Regarding the maturity ogive analysis, TL at 50% maturity was found to lay at 180.1 cm, and 90% maturity at 199.0 cm (Fig. 3). Testicles weight ranged from 84.4 to 92.2 g in males stage 2 (et al. n = 3) and from 158.4 to 352.8 g in adults (et al. n = 4).
A total of 46 immature females were captured ranging from 104.0 to 230.0 cm TL. There was only one stage-2 (204.0 cm TL), and no stage-3 females in record. Eleven specimens had their oviduct width measured, presenting values between 4.1 and 7.15 mm.
Population structure
A total of 83 individuals were captured and measured over the six fishing cruises and four scientific cruises (Table 2), with number of individuals varying between 1 and 26 per cruise. The TL range for the total sample varied from 104.0 to 270.0 cm (Fig. 4), with a mean of 165.7 cm. Altogether, 37 males were captured, TL ranging between 119.0 and 270.0 cm, with a mean of 177 cm, while the 46 females captured had TL values between 104.0 and 230.0 cm, with a mean of 156.6. The smallest individual was 104.0 TL female, captured in July 2015, at 20.8°C and 150 m depth, while the larger individual was a 270.0 cm male, captured in March 2015 at 24.9ºC surface temperature and 2000 m depth.
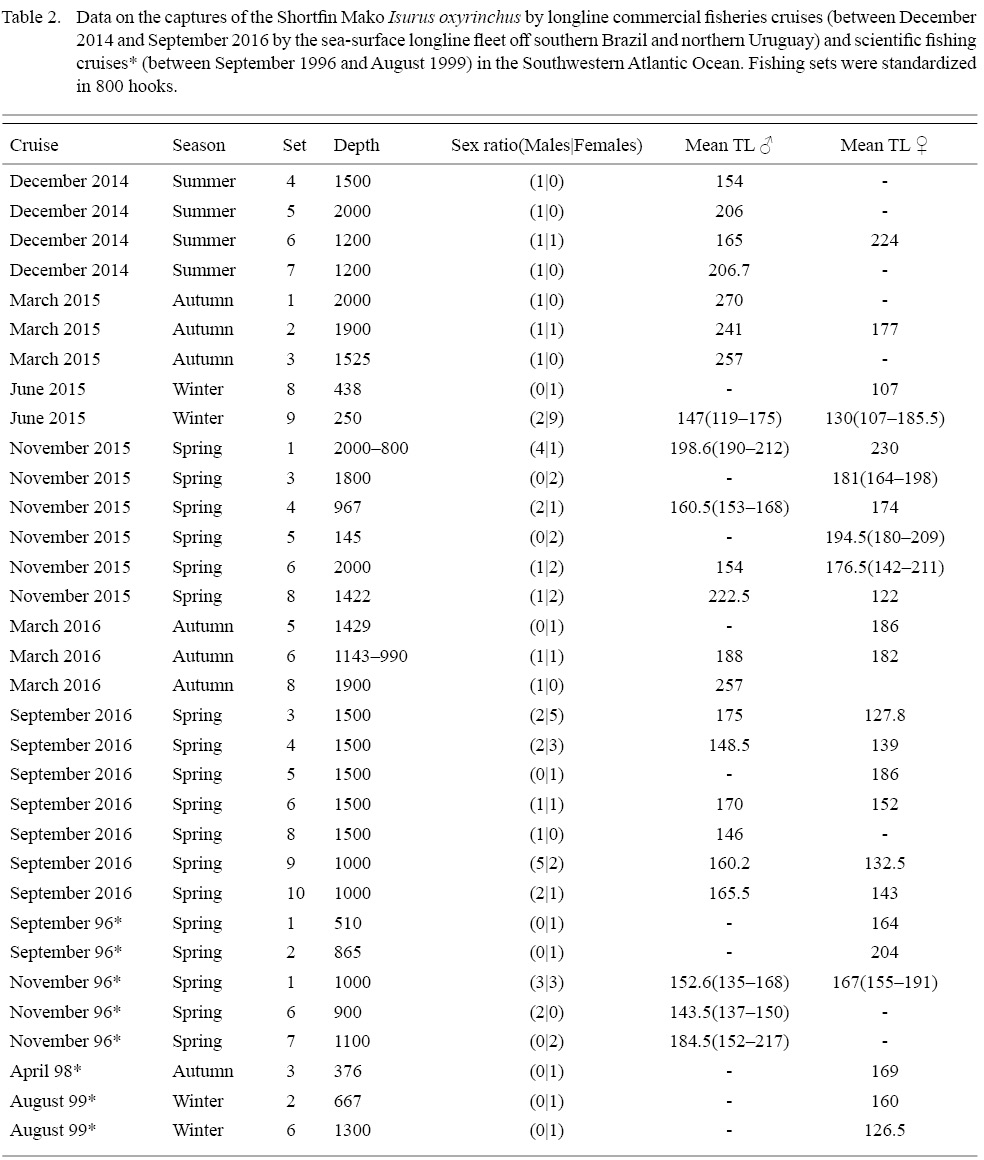
Table 2
In relation to the CPUE, the highest value was recorded in the Spring sets, which presented 2.83 individuals per 1000 hooks, followed by Winter (1.67), Autumn (0.74) and Summer (0.57). The mean TL was smaller in the Winter sets (134), increasing in Spring (163), Summer (191) and Autumn (214).The largest individuals were captured in the March 2015 Cruise (Autumn), with the samples varying between 177.0 and 270.0 cm, and composed almost exclusively by males larger than 240.0 cm, with the exception of a 177.0 cm TL female, whilst the smaller individuals were recorded in the June 2015 cruise (Winter), with captures composed mostly by females smaller than 160.0 cm. The CPUE by season and maturity stage is presented in Figs. 5, 6 and 7. The overall sex ratio, 1.24:1, favoured females, was statistically significant (χ2 = 0.38, p = 0.53), and was highly variable among seasons. The higher F:M ratio was registered in the winter sets (٦:١), decreasing in Spring (1.12:1), and favouring males in Autumn (0.8:1) and Summer (0.25:1).
Discussion
Constituting the first estimate for the SW Atlantic Male TL at 50% maturity was estimated at 180 cm. For other areas of the Atlantic, Stevens (1983) and Maia et al. (2007) had provided estimations for the NW and NE Atlantic, respectively. Our estimate falls within the range of values observed elsewhere (from 150 to 180 cm TL) (Table 3). Our ogive analysis, although based on a relatively small sample of immature juveniles (19 in all), showed the expected sigmoidal curve and size at maturity for males, congruent with other studies. Compared to other clasper length-TL studies, immature males in the range of 50–100 cm are missing from the present study (see for instance, Conde-Moreno and Galván-Magaña, 2007; Semba et al. , 2011). As only immature females were captured, maturity estimates are not available in this study. Given the effort concentrated in the same area, close to the Brazil-Uruguay Economic Exclusive Zone southernmost border, our data suggests size segregated groups, varying seasonally, with larger individuals concentrating northwards, in warmer waters and immature and juveniles migrating southwards as they grow.
Sampling oceanic and pelagic sharks is an expensive and difficult task, requiring many resources and structure, rarely allowing scientific cruises to be undertaken, thus leaving the assessment of their biological traits to fishery-dependent means (Baumet al. , 2003, Barreto et al. , 2016a). In the specific case of the Shortfin Mako, access to fish for biological sampling is even more problematic. Its valuable meat may discourage commercial skippers from allowing sampling (Barreto et al. 2016b). Fishery-dependent sampling tends to concentrate efforts in areas of aggregation of other target species, varying in depths and areas. However, these snapshots can yield important information.

In winter months, the fleet sets its fishing gear in southernmost waters less than 500 m depth, catching mostly immature females and eventually juvenile males, while in the summer, autumn and spring months, the fishing operations were conducted in waters deeper than 800 m, catching mostly adult and juvenile males, and occasionally immature females. These trends in distribution may provide information on spatial distribution and abundance of genders and maturity classes. Our data indicates mainly juvenile and immature aggregations with occasional presence of adult males in Southern Brazil pelagic waters. Vooren et al.(2005) reported neonates and young-of-the-year captures by gillnet fishery between January and March, in shallow coastal waters (28 to 63 m depth) between 29 and 31°S, classifying the area as a nursery in the summer. Costa et al. (2002), analyzing pregnant females captured between September and February by longline tuna fishing vessels, northwards, between 20–28°S, concluded that the birth period in Southeastern Brazil occurs in Spring (October to December). Migratory capacity among young-of-the-year and neonates is reported by Maia et al., (2007), and may explain our pattern observed in capture sizes observed, with the individuals going southwards, to feeding areas.
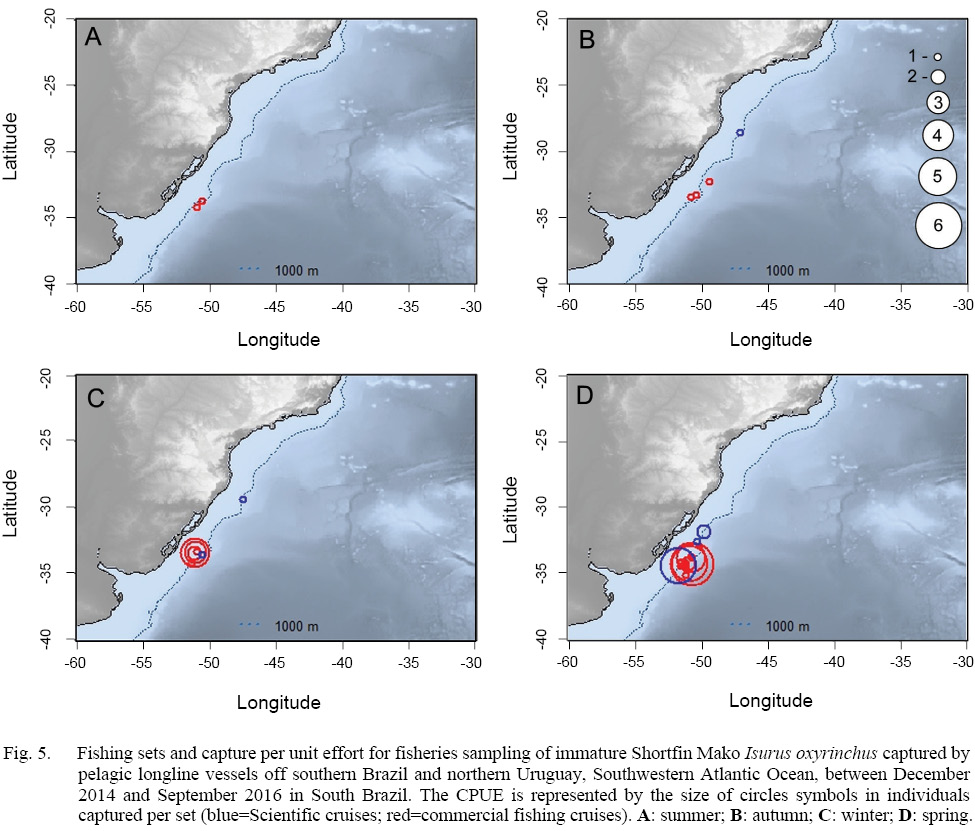
Fig. 5
The high migratory capacity and behavior of this species widely exposes its populations to a variety of fishing fleets and techniques, with young and neonates being captured by gillnet fisheries in shallower water closer to the continent and larger individuals being captured by more oceanic techniques such as longlines (Vooren et al. , 2005; Costa et al. 2002; personal observations). This exposure makes the Shortfin Mako the second most captured shark in the South Atlantic, with immature individuals being the most exposed to fisheries (Barreto et al., 2016a). Tsai et al. (2014) showed by modeling sex-specific reproductive stocks that single-sex models could be biased, leading to wrong risk analyses and population estimates. Not only the capture of females (from all size classes) is detrimental to the population, captures of the male stock may lead to population depletion, depriving it from viable reproductive pairs (Tsai et al. , 2014).
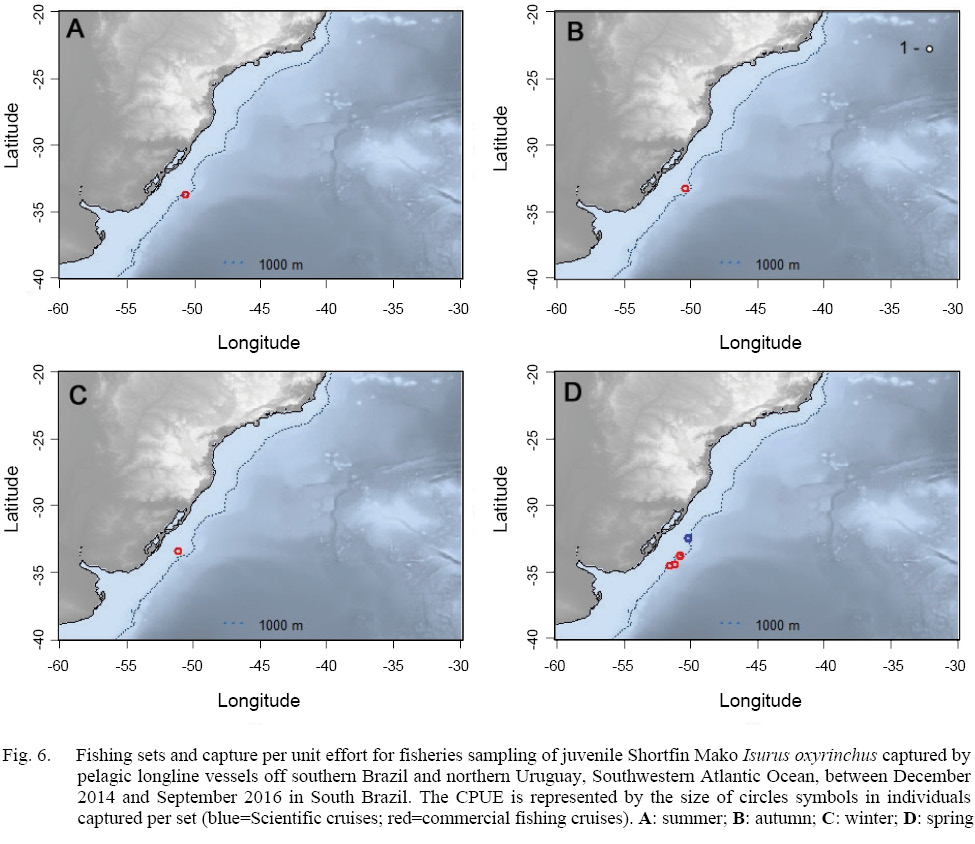
Fig. 6
Genetic studies have suggested that the North and South Atlantic Shortfin Mako populations are not segregated, having some gene flow between them (Schrey and Heist, 2003). Nevertheless, female reproductive traits reviewed by Mollet et al. (2000) differ between hemispheres, which could be explained by habitat variation, with different food availability, for example. This situation signifies a discrete management approach, rather than to extrapolate trends and patterns from one hemisphere to the other.
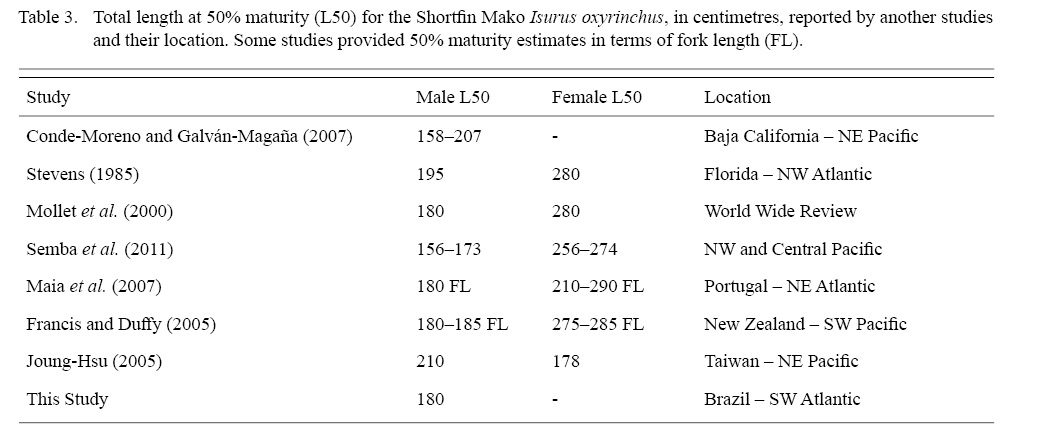
Table 3
Conservation decisions and laws have changed in Brazil in the last few years, as stated by Barreto et al.(2016a), with the prohibition on capturing Sphyrnids (any kind of landing, even as bycatch), among other threatened sharks. The changes in fishing laws have led to modifications of the fishing areas which had become shallower, and closer to nursery areas. These are important areas for fishery management, providing a greater contribution for recruitment than other areas (Beck et al., 2001). Monitoring captures in these areas is necessary. Immature individuals are not able to reproduce, and their capture may lead to future reduction of the reproductive stock and therefore reducing the population resilience, especially in slow growth and low fecundity species, as the Shortfin Mako. However, proper management should not focus on a given maturity stage. All stages should be subject of concern when conservation decisions are made. More detailed studies are necessary, quantifying not only number of individuals or biomass captured, but also length, age and maturity class distributions, allowing for a responsible and sustainable management of fisheries, conserving the stocks and the economic viability of fisheries.
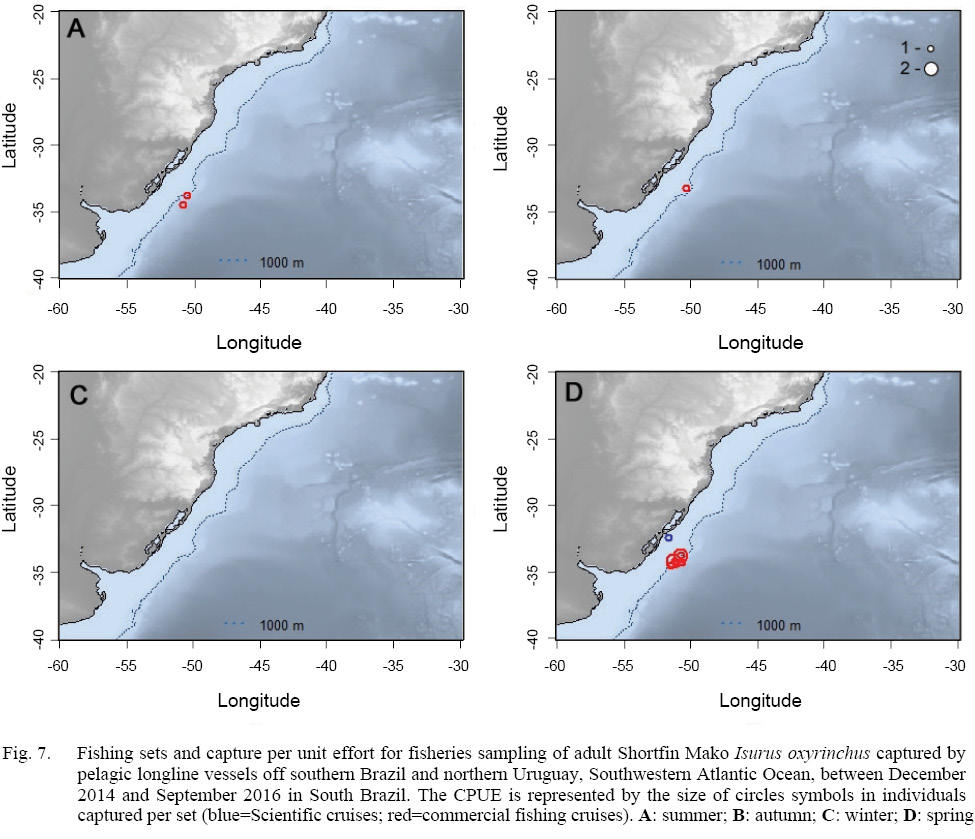
Fig. 7
Acknowledgements
We are very grateful to those who were involved in the realization of this research, particularly to the skippers for their receptivity and solicitude in receiving us on their boats. We also wish to thank the Instituto de Oceanografia/FURG, for sharing the Project ARGOS data, and to Projeto Albatroz, specially to Augusto Costa, for the logistical support. This project was funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) with a master’s degree scholarship.
References
Barreto, R. R., Ferretti, F., Flemming, J. M., Amorim, A. F., Andrade, H., Worm, B. and Lessa, R. 2016a. Trends in the exploitation of South Atlantic shark populations. Conservation Biology, 30: 792–804. https://doi.org/10.1111/cobi.12663
Barreto R. R., Farias, W. K. T., Andrade, H., Santana, F. M. and Lessa, R. 2016b. Age, growth and spatial distribution of the life stages of the shortfin mako, et al. Isurus oxyrinchus (Rafinesque, 1810) caught in the Western and Central Atlantic. PLoS ONE 11(4): e0153062. https://doi.org/10.1371/journal.pone.0153062
Baum, J. K., Myers, R. A., Kehler, D. G., Worm, B., Harley, S. J. and Doherty, P. A. 2003. Collapse and conservation of shark populations in Northwest Atlantic. Science, 299: 389–392. https://doi.org/10.1126/science.1079777
Beck, M.W., Heck, K. L., Able, K. W. Childers, D. L., Eggleston, D. B., Gillanders, B. M., Halpern, B., Hays, C. G., Hoshino, K., Minello, T. J., Orth, R. J., Serdan, P. F. and Weinstein, M. P. 2001. The Identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. BioScience, 51: 633–641. https://doi.org/10.1641/0006-3568(2001)051[0633:TICAMO]2.0.CO;2
Cailliet G. M, Martin L. K., Harvey, J. T., Kusher, D. and Welden, B. A. 1983. Preliminary studies on the age and growth of the blue shark, et al. Prionace glauca, common thresher, et al. Alopias vulpinus, and shortfin mako, et al. Isurus oxyrinchus, from California waters. US Department of Communication, NOAA Technical Reports, NMFS 8: 179–188.
Carey, F. G., and Teal, J. M. 1969. Mako and porbeagle: warm-bodied sharks.et al. Comparative Biochemistry and Physiology, 28(1), 199–204. https://doi.org/10.1016/0010-406X(69)91335-8
Casey, J. G. and Kohler, N. E. 1992. Tagging studies on the shortfin mako shark (et al. Isurus oxyrinchus) in the western North Atlantic. Australian Journal of Marine and Freshwater Research, 43: 45–60. https://doi.org/10.1071/MF9920045
Compagno, L. J. V. 1984. FAO species catalogue: sharks of the World: an annotated and illustrated catalogue of shark species known to date. FAO Fisheries Synopsis, 4(1–2): 665p.
Compagno, L. J. V. 1990. Alternative life history styles of cartilaginous fishes in time and space. Environmental Biology of Fishes, 28: 33–75. https://doi.org/10.1007/978-94-009-2065-1_3
Compagno, L. J. V. 2005. Checklist of living Chondrichthyes. et al. In: W. Hamlett (Ed.), Reproductive Biology and Phylogeny of Chondrichthyes: Sharks, Batoids and Chimaeras, pp. 503–548. Science Publishers, Inc., Enfield, New Hampshire, USA.
Conde-Moreno, M., and Galván-Magaña, F. 2006. Reproductive biology of the mako shark et al. Isurus oxyrinchus on the south-western coast of Baja California, Mexico. Cybium, 30(4): 75–83.
Cortés, E., Arocha, F., Beerkircher, L., Carvalho F., Domingo, A., Heupel, M., Holtzhausen, H., Santos, M.N., Ribera, M., Simpfendorfer, C. 2010. Ecological Risk Assessment of Pelagic Sharks Caught In Atlantic Pelagic Longline Fisheries. Aquatic Living Resources, 23: 25–34. https://doi.org/10.1051/alr/2009044
Costa, F. E. S., Braga, F. M. S., Arfelli, C. A., and Amorim, A. F. 2002. Aspects of the reproductive biology of the Shortfin mako, et al. Isurus oxyrinchus (elasmobranchii Lamnidae), in the southeastern region of Brazil. Brazilian Journal of Biology, 62(2): 239–248. https://doi.org/10.1590/S1519-69842002000200007
Francis, M. P., and Duffy, C. 2005. Length at maturity in three pelagic sharks (et al. Lamna nasus<, et al. Isurus oxyrinchus, and et al. Prionace glauca) from New Zealand. Fishery Bulletin, 103(3): 489–500.
Garcia, C. E. 1997. Physical oceanography. et al. In: U. Seeliger, C. Odebrecht, and J. P. Castello (Eds.), Subtropical convergence environments. pp 94–96. The coast and sea in the southwestern Atlantic. Springer, Berlin Heidelberg New York.
Gilmore, R. G. 1993. Reproductive biology of Lamnoid sharks. et al. In: L. S. Demski and J. P. Wourms, (Eds.), The reproduction and development of sharks, skates, rays and ratfishes. Environmental Biology of Fishes, 38(1–3): 95–114. https://doi.org/10.1007/BF00842907
Gilmore, R. G. 2005. Oophagy, intrauterine canibalism and reproduction strategy in Lamnoid Sharks. et al. In W. Hamlett, (Ed.), Reproductive Biology and Phylogeny of Chondrichthyes: sharks, batoids and chimaeras. pp 435–462. Science Publishers, Inc., Enfield, New Hampshire, USA.
Hamlett, W. C. and Koob, T. J. 1999. Female reproductive system. et al. In: W. Hamlett, (Ed.), Sharks, Skates, and Rays. The Biology of Elasmobranch Fishes. pp. 398–443. Baltimore, MD. The John Hopkins University Press.
Holden, M. J. 1974. Problems in the rational exploitation of elasmobranch populations and some suggested solutions. et al. In: F. R. Harden-Jones (Ed.) Sea fisheries research. pp. 117–137. John Wiley and Sons, New York.
Joung, S., and Hsu, H. 2005. Reproduction and embryonic development of the shortfin mako, et al. Isurus oxyrinchus Rafinesque, 1810, in the Northwestern Pacific Zoology Studies, 44(4): 487–496.
Maia, A., Queiroz, N., Cabral, H. N., Santos, A. M., and Correia, J. P. 2007. Reproductive biology and population dynamics of the shortfin mako, et al. Isurus oxyrinchus Rafinesque, 1810, off the southwest Portuguese coast, eastern North Atlantic. Journal of Applied Ichthyology, 23: 246-251 https://doi.org/10.1111/j.1439-0426.2007.00849.x
Mollet, H. F., Cliff, G., Pratt Jr. H. L., and Stevens, J. D. 2000. Reproductive biology of the female shortfin mako,et al. Isurus oxyrinchus Rafinesque, 1810, with comments on the embryonic development of lamnoids. Fishery Bulletin, 98: 299–318.
Mollet, H. F., Testi, A.D., Compagno, L. J. V. and Francis, M. P. 2002. Re-identification of a lamnid shark embryo. Fishery Bulletin, 100: 865–875.
Mourato, B. L., Arfelli, C. A., Amorim, A. F., Hazin, H. G., Carvalho, F. C., and Hazin, F. H. V. 2011. Spatio-temporal distribution and target species in a longline fishery off the Southeastern Coast of Brazil. Brazilian Journal of Oceanography, 59(2):185–194. https://doi.org/10.1590/S1679-87592011000200007
Pratt H. L. Jr., and Casey J. G. 1983. Age and growth of the shortfin mako, et al. Isurus oxyrinchus, using four methods. Canadian Journal of Fisheries and Aquatic Science, 40: 1944–957. https://doi.org/10.1139/f83-224
R Core Team (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org/.
Rigby, C. L., Barreto, R., Carlson, J., Fernando, D., Fordham, S., Francis, M. P., Jabado, R. W., Liu, K. M., Marshall, A., Pacoureau, N., Romanov, E., Sherley, R. B. and Winker, H. 2019. et al. Isurus oxyrinchus. The IUCN Red List of Threatened Species 2019: e.T39341A2903170. https://dx.doi.org/10.2305/IUCN.UK.2019-1.RLTS.T39341A2903170.en. Downloaded on 20 October 2020.
Schrey, A.W. and Heist, E. J. 2003. Microsatellite analysis of population structure in the shortfin mako (et al. Isurus oxyrinchus) et al. Canadian Journal of Fisheries Science, 60: 67–675 https://doi.org/10.1139/f03-064
Semba, Y., Ichiro, A., and Kotaro, Y. 2011. Size at maturity and reproductive traits of shortfin mako, et al. Isurus oxyrinchus, in the Western and Central North Pacific. Marine and Freshwater Research, 62: 20–29 https://doi.org/10.1071/MF10123
Stevens, J. D. 1983. Observations on reproduction in the shortfin mako et al. Isurus oxyrinchus. Copeia: 126–130. https://doi.org/10.2307/1444706
Stevens, J. D. 2008. The Biology and Ecology of the shortfin mako shark, et al. Isurus oxyrinchus. et al. In: M. D. Camhi, E. K. Pikitch and E. A. Babcock (Eds.), Sharks of the Open Ocean: Biology, Fisheries and Conservation. pp 260–267. Blackwell, Oxford. https://doi.org/10.2307/1444706
Tsai, W., Liu, K., Punt, A. E. and Sun, C. 2014. Assessing the potential biases of ignoring sexual dimorphism and mating mechanism in using a single-sex demographic model: the shortfin mako shark as a case study. ICES Journal of Marine Science, 72(3): 793–803. https://doi.org/10.1093/icesjms/fsu210
Vooren, C. M., Klippel, S., and Galina, A. B. 2005. Elasmobrânquios das águas costeiras da Plataforma Sul. In: C. M. Vooren and S. Klippel (Eds.), Ações para a conservação de tubarões e raias no sul do Brasil. pp. ١١٣–١٢٠. Porto Alegre: Igaré.
Wyffels, J. T. 2009. Embryonic development of chondrichthyan fishes: a review. et al. In: Y.et al. Kunz, B. G. Kapoor, and C. A. Lauer (Eds.), Development of Non-Teleost Fishes. pp. 1–103. Science Publishers Inc., Enfield. https://doi.org/10.1201/b10184-2
Citation: Canani, G. and Oddone, M.C. 2020. Reproductive biology of
Isurus oxyrinchus captured by the south
Brazilian surface longline commercial fleet in the Southwest Atlantic Ocean, with data on CPUE and size distribution by sex. J. Northw. Atl. Fish. Sci., 51: 105–116.
https://doi.org/10.2960/J.v51.m724