Introduction
Multi-species interactions as a component of ecosystem approaches are becoming more important in fisheries management, including ecosystem modelling. Large mobile animals, such as whales, seasonally migrate to a feeding area, however the spatial and temporal aspect of the feeding habits of higher trophic predators remains largely undocumented in the western North Pacific. Furthermore the effects of environmental variability on their distribution and induced feeding habits are becoming important for understanding the ecosystem and development of habitat modelling (Redfern et al., 2006). To precisely understand the feeding habits in relation to the various factors, large-scale survey including the variation of geographical and temporal information in targeted species is necessary.
The offshore waters of the Pacific coast of Japan are important fishing grounds (Shiomoto et al., 1994; Kasai et al., 1997) with high productivity (Olson, 2001). Marine mammals such as seals, sea lions, and whales, occur commonly in these waters and are important components of the ecosystem. Baleen whales, in particular, are presumed to be important due to their large biomass and prey requirements (Trites et al., 1997; Leaper and Lavigne, 2007) when they seasonally migrate to foraging areas (Nemoto, 1959; Masaki, 1976; Hatanaka and Miyashita, 1997). Common minke (Balaenoptera acutorostrata, hereafter referred to as minke whale), sei (B. borealis) and Bryde’s (B. edeni) whales are commonly found balaenopterid species and were harvested during the commercial whaling period. To understand the role of baleen whales in the ecosystem, and to better manage fisheries at the ecosystem level through multispecies modelling, an examination of their spatio-temporal feeding habits and the extent of consumption in a given area are necessary. Additionally, the effect of environmental factors on feeding habits must be quantified coincidentally to interpret the results properly.
The feeding habits of large baleen whales were examined previously based on samples from commercial whaling (Nemoto, 1959; Nemoto and Kawamura, 1977; Kawamura, 1982), however these studies were mainly qualitative in nature. Furthermore, the information from those reports is difficult to extrapolate to the present because of different areas covered and changes in the marine environment. The Japanese Government carried out the Japanese Whale Research Program under Special Permit in the western North Pacific (JARPN) from 1994 to 1999. This program provided both qualitative and quantitative data on the feeding ecology of minke whales. The results showed that the minke whale feed on many prey types ranging from small crustaceans to large fish (Tamura et al., 1998; Tamura and Fujise, 2002), and many of these prey are also targeted by Japanese fisheries. The Second Phase of JARPN (JARPN II) expanded sampling to other whales such as sei, Bryde’s, and sperm whales (Physeter macrocephalus), which are species with a large biomass and likely play an important role in the ecosystem. The JARPN II program also has broader spatial coverage than JARPN.
The purposes of this study were to (1) investigate the feeding ecology of minke, sei, and Bryde’s whales by examining the diets and overlap of diets among these species, (2) examine the factors that may influence the features of the whales' feeding habits and distribution, and (3) estimate the amount of prey consumed by the three whale species. To estimate prey consumptions by whales in these waters, we incorporated diet data for these whales, together with recent numbers of whales in the study area based on dedicated sighting surveys undertaken as part of JARPN II.
Materials and Methods
Study area
The research area of JARPN II was the area between the Pacific coast of Japan and 170° E, and a latitudinal range between 35º N and 50° N (Fig. 1), including a part of sub-areas 7, 8 and 9 established by the Scientific Committee of the International Whaling Commission for management purposes (IWC, 1994). This area has a characteristic physical environment in which the cold Oyashio current from the north and warm Kuroshio current from the south meet and alter their direction offshore, making the Kuroshio-Oyashio transition region (Fig. 1; Olson, 2001). The Kuroshio current transports eggs and juveniles of commercially important Japanese sardine (Sardinops melanostictus), Japanese anchovy (Engraulis japonicus), Pacific saury (Cololabis saira), and mackerels (Scomber spp.) to pelagic waters (Takahashi et al. 2001; Takahashi and Watanabe, 2004; Itoh and Kimura, 2007; Watanabe, 2009). The pelagic transition region is also an important feeding ground for the growth and survival of these fish (Takahashi and Watanabe, 2004; Itoh and Kimura, 2007).
Sampling and sighting survey
A total of 740 minke and 393 Bryde’s whales were sampled by JARPN II from May to September during 2000–2007. A total of 489 sei whales were also sampled from 2002–2007 (Table 1). The locations of the sampled whales and sea surface temperature (SST) were recorded, and the whales were transported to a research vessel where they were examined by biologists. All whales were measured, weighed, and their reproductive organs were examined to distinguish sexual maturity. Males of minke, Bryde's, and sei whales, were defined as sexually mature by testis weights (larger side) of more than 290 g, 560 g and 1 090 g, respectively (Bando et al., pers. comm., Institute of Cetacean Research, Toyomi 4-5, Chuo-ku, Tokyo 104-0055, Japan). Females were defined as sexually mature by the occurrence of at least one corpus luteum or albicans in their ovaries.
Dedicated sighting surveys were also conducted as part of JARPN II through 2000–2007 for the purpose of estimating whale abundance estimates. Effort and primary sighting on the designed survey track lines were recorded.
Stomach contents
Baleen whales have four-chambered stomachs (Hosokawa and Kamiya, 1971; Olsen et al., 1994). Since newly-eaten prey were found commonly in both the first (forestomach) and second (fundus) stomach compartments, we only included contents from these two compartments in the analyses. Stomach contents were removed from each chamber and a part of the contents were sub-sampled after weighing the total contents to the nearest 0.1 kg. If fresh prey were found in the stomach, their body lengths were measured for up to 100 samples from each whale.
In the laboratory, intact prey and remains such as fish otoliths and crustaceans were identified to the lowest possible taxon using gross morphological characteristics (Tamura and Fujise, 2002). The total weight of each prey species before ingestion in the subsample was then estimated by multiplying the average weight of fresh specimens by the estimated number of individuals based on remains in the stomach.
Diet and environmental factors
To examine the spatial and temporal variation of whale diets in relation to seasonal SST patterns, the positions where whales whose stomachs contained main prey items were plotted on the satellite images of SST obtained from Ocean Color Web (http://oceancolor.gsfc.nasa.gov/), using overlay function of GIS software (Marine Explorer: Environmental Simulation Laboratory, Japan).
Canonical correspondence analysis (CCA), which is categorised as one of the habitat models (Redfern et al., 2006), is a powerful tool that can identify features within diet compositions and habitat use together with biological and environmental factors (Guisan and Zimmermann, 2000; Redfern et al., 2006), and this analysis has been used in other whale studies (Fielder and Reilly, 1994; Reilly and Fiedler, 1994; Haug et al., 2002).
The CCA was performed on diet composition and environmental factors (SST, year, date, latitude, and longitude) and biological factors (body length and sex of whales) in each whale species, using the ‘vegan’ package (ver.1.13-1, Oksanen et al., 2008) in R (http://www. r-project.org). Each environmental variable was tested by 1 000 permutations and considered significant at the 5% level.
Distribution and environmental factors
CCA was also used to examine whale habitat use by the three whale species in relation to environmental variables. We assumed that a species response, such as abundance or density, reflects the habitat suitability of environmental conditions at that site. Whale density was calculated as the whale number detected per 1 nautical mile (nm) on the survey track line from the sighting survey, and transformed 1 × 1 degree gridded data by month and year. In this analysis, gridded data with small effort (≤1 nm) per a grid were eliminated to avoid biases. Monthly SST and chlorophyll a concentration 9 x 9 km data in July to September in 2000–2007 obtained from Ocean Color Web were also transformed to gridded data in the same manner as for the whale diet analyses above. Then, the CCA was performed on whale densities and environmental factors, i.e., SST, chlorophyll a, year, month, latitude, and longitude.
Diet overlap
To determine the amount of diet overlap among whale species, Pianka’s index of niche overlap (Pianka, 1973, 1974) was calculated between the reconstructed prey weights of whales by sub-areas as follows:
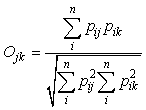
where Ojk is Pianka’s measure of overlap, pij is the proportion that diet item i is of the total prey used by species j, pik is the proportion that prey i is of the total prey used by species k, and n is the total number of diet items. Theses calculations was done using ‘pgirmess’ package (ver. 1.3.8, Giraudoux, 2008) in R and the index value was bootstrapped 1000 times to reduce bias.
Estimating prey consumption
The daily food consumption by the three whale species was estimated based on mass-specific energy requirement estimates (e.g., Sigurjónsson and Víkingsson, 1997). The modelled prey biomass values were then apportioned to each prey species based on the proportional contribution of prey species from the stomach contents. To consider the spatio-temporal variation in prey distribution, the prey consumption was derived for each sub-area (7, 8 and 9), period (early period: May and June, late period: July to September), and whale maturity stage (immature and mature), as energy requirements differ among sexual maturity classes (e.g., Lockyer, 1987; Víkingsson, 1995). Finally, the total annual consumption in the study area by these three whale species throughout their feeding period was estimated for each prey species.
Daily prey consumption. The daily consumption of each prey species (Dkg) was calculated by the following equations (Sigurjónsson and Víkingsson, 1997):
Dkg = Dkcal /Ekcal
Dkcal = 206.25M 0.783
where Dkg is daily prey mass consumption by whales (kg day-1),Dkcal is daily prey energy consumption (kcal day-1), Ekcal is the mass-specific caloric value of prey species (kcal kg-1), and M is mean body weight of whales (kg).
In this study the wet mass caloric values of dominant preys, i.e., copepod (Neocalanus cristatus: 920 Kcal kg-1), euphausiid (Euphausia pacifica: 850 Kcal kg-1), Japanese anchovy (small size: 1 320 Kcal kg-1, large-size 1 530 Kcal kg-1), Pacific saury (3 140 Kcal kg-1), walleye pollock (1 490 Kcal kg-1) and Japanese flying squid (Todarodes pacificus: 1 580 Kcal kg-1) (T. Tamura, pers. comm., Institute of Cetacean Research, Toyomi 4-5, Chuo-ku, Tokyo 104-0055, Japan ), were used to estimate wet prey composition. We assumed that other minor prey species had the same caloric density as Japanese anchovy. Then Ekcal was estimated based on prey composition from stomach contents analysis for each sub-area, per month.
Prey consumption by whales for the feeding period. Lockyer (1981) indicated that 83% of the annual energy intake of balaenopterid species is ingested during the summer, corresponding to approximately ten times higher feeding rates during summer feeding period than during the rest of the year.
Based on this assumption, the average daily prey consumption (SDkg) during this feeding period (May to September) was estimated for these baleen whales using the following equation:
SDkcal= 2.020Dkcal
We calculated daily prey consumption by whales using SDkcal and relative diet composition (percent wet weight) in each area and month. Finally, we calculated prey consumption by these whales based on the assumption that the minke, sei, and Bryde’s whales spend about 150 days (feeding period) in this feeding area for each sub-area, and sexual maturity category.
The number of whales present in the study area was estimated by dedicated, vessel-based sighting surveys as part of JARPN II (Hakamada et al., MS 2009; Table 2). To consider changes in the seasonal abundance of these whales, the numbers of whales were estimated in both early (May and June) and late (July to September) survey periods.
Results
Diet of whales in the study area
Minke whales. A total of 14 prey species, including one copepod, two euphausiids, two squids and 10 fishes were identified in the 740 minke whale stomachs (Table 3). Japanese anchovy and Pacific saury were the most frequently occurring prey in the stomachs. Almost all (88%) of the stomachs contained a single prey species; 11% of the stomachs contained two species, while only 0.6% contained more than two prey species.
The modal body size of the anchovy consumed ranged from 70 to 150 mm (mode = 130 mm) (Fig. 2A). The body size of Pacific saury consumed ranged from c. 150 to 350 mm (mode = 300 mm) (Fig. 3A). Minke whales in this study area also fed on large-sized mackerels (Scomber spp.; Fig. 4A), but they consumed relatively few fish of this size.
Sei whales. A total of 12 prey species, including two copepods, three euphausiids, one squid and five fish species were identified in the 489 sei whale stomachs examined. Copepods, euphausiids and Japanese anchovy occurred most frequently (Table 3). Most of the copepods found in the stomachs were 5th copepodite stage of Neocalanus cristatus and N. plumchrus. Most of these stomachs (90%) contained one prey species, and the rest contained two species in the stomachs. The size of the anchovy consumed by the sei whales ranged from 25 to 140 mm (mode = 130 mm) (Fig. 2B). The size of Pacific saury consumed ranged from c. 110 to 350mm, with two modes at 220 and 300 mm (Fig. 3B). The sei whales also fed on small-sized mackerel consisting of both chum mackerel (Scomber japonicus) and spotted mackerel (S. australasicus) ( Fig. 4).
Bryde’s whales. A total of 18 prey species, including five euphausiids, one squid and 12 fish species were identified in the 393 Bryde’s whale stomachs (Table 3). Most of these stomachs (92%) contained a single prey species and the rest contained two prey species. The modal size of the anchovy consumed was 70 mm ranging from 20 to 140 mm (Fig. 2C). Bryde’s whales also fed on small-size mackerels ( Fig. 4C).
Diet niche overlapping among whales. The values of Pianka’s measure of overlap showed the diet niche overlapping among whale species in the study area (Table 4), showing the diet of minke and sei whales was highly overlapped in most coastal sub area (Sub-area 7: >0.8) and being lower in offshore waters (Sub-area 8 and 9; 0.4–0.3). A similar trend was also seen in the case of minke and Bryde’s whales. The diet overlap of sei and Bryde’s whales was high through small areas (0.7–0.9), suggesting these two species share a similar diet niche.
Diet of whales with environmental and biological variations in SST in the study area
Minke whale. The surface temperatures where minke whales occurred ranged from 8 to 18°C, with a mode at 12°C (Fig. 5). The minke whales fed mainly on anchovy around 40° N in May and June, after which the whales moved northward where they fed mostly on Pacific saury in July to September around 45° N (Fig. 6).
Of seven variables used in the CCA, all but sex significantly contributed to explaining the composition of minke whale’s diet, i.e., year, date, latitude, longitude, SST and body length (Fig. 7). The eigenvalues of the first and second canonical axes were 0.497 and 0.176 and accounted for 78% of all axes. The positive canonical axis 1 represented lower SST, large body size, later season, high latitude and eastern area, and positive axis 2 represented later year, lower temperature and later season. Prey similarity to the factors divided saury and squid, mackerel and copepod, euphausiid and anchovy, into different groups. The importance of anchovy increased with SST and decreased with body length, latitude, longitude and date, and that of saury tended to increase with body length, latitude, longitude and date, and decrease with SST. The importance of mackerel and copepod increased with year and SST and decreased with date.
Sei whale. Sei whales occurred in pelagic waters with surface temperatures ranging from 10 to 24°C, with a mode of 14°C (Fig. 5). Their main prey, euphausiids, copepods, and Japanese anchovy, were found throughout the area of this predator’s distribution (Fig. 8).
Of seven variables used in CCA, all but sex and body length significantly contributed to explaining the composition of the diet of sei whale, i.e., year, date, latitude, longitude, and SST (Fig. 9). The eigenvalues of the first and second canonical axes were 0.355 and 0.131 and accounted for 84% of all axes. The positive canonical axis 1 represented higher SST and western area, and positive canonical axis 2 represented later year, higher SST, lower latitude and early season. The importance of anchovy was high with higher SST and western area, copepod with high longitude, mackerel with later year and warmer SST.
Bryde’s whale. Bryde’s whales occurred at the southern part of the study area with surface temperature ranging from 17 to 27°C, with a mode of 23°C (Fig. 5). Euphausiids and Japanese anchovy were the dominant prey species. Euphausiids were the dominant prey species early in the feeding period at longitude 145º E, while Japanese anchovy was dominant from July to September (Fig. 10).
Of seven variables used in CCA, year, date, longitude, and SST contributed significantly to explaining the composition of Bryde’s whale’s diet (Fig. 11). The two canonical axes were created in the CCA, and the eigenvalues of these first and second canonical axes were 0.317 and 0.06. The positive canonical axis 1 represented later season, higher SST, later year, and the positive canonical axis 2 represented early year, later season and western area. Anchovy and euphausiids were plotted on the other direction of on the first canonical axis, and these two preys were separated by the second canonical axis. This result indicated that anchovy and euphausiids were eaten by Bryde’s whales in different quality environments. Mackerel were eaten more in later year and eastern area than other prey.
Whale distribution pattern in relation to environmental factors
Of six variables used in CCA, SST, chlorophyll a, latitude and longitude, contributed significantly to explaining the composition of whale density, while year (p = 0.069) and month (p = 0.463) did not significantly contribute (Fig. 12). Positive canonical axis 1 represented warmer SST, lower latitude and low chlorophyll a concentration, and positive score canonical axis 2 represented western area and high chlorophyll a concentration.
The density of minke whale was explained by high chlorophyll a concentration, high latitude, western area and lower SST. The density of sei whales was explained by SST, latitude (between minke and Bryde’s whales), and most western area. The density of Bryde’s whale was clearly explained by the first axis, suggesting higher SST and lower latitude.
Prey consumption by whales during the feeding period
Estimated total prey consumption per prey type for each whale species is shown in Table 5, and the standing biomass and catches are shown in Table 6. The total prey consumption by the three whales was estimated to be c. 1.6 million t. Sei whales, which are most abundant (Hakamada et al., MS 2009: Table 2), are by far the most important predator in the study area (c. 911 000 t), followed by the Bryde’s (c. 565 000 t) and minke whales (c. 160 000 t).
The weight of Japanese anchovy consumed by minke, sei and Bryde’s whales was c. 774 000 t (range: 318 000–2 146 000 t) in the study area, which was more than twice the recent total commercial catch, and equivalent to 16.1% of the anchovy’s total biomass of 4.8 million t (Fisheries Agency of Japan, 2008). The largest consumer of the anchovy among the baleen whales in the study area was Bryde’s whale followed by sei and minke whales (Table 5).
The weight of Pacific saury consumed by only minke and sei whales was c. 44 000 t (range 17 000–235 000 t), equivalent to approximately 10.7% of the total recent catch (Fisheries Agency of Japan,
2008). The weight of mackerels consumed by the whales was c. 140 000 t (range: 67 000–218 000 t), mostly consumed by sei whales, equivalent to approximately 32.8% of the total recent mackerel catch and 13.8% of their biomass (Fisheries Agency of Japan, 2008).
Copepods and euphausiids consumed by the whales were c. 275 000 t (range: 136 000–562 000 t) and c. 363 000 t (range: 146 000–1 208 000 t), respectively. The largest consumer of both copepods and euphausiids were sei whales, although the higher end of the range of euphausiid consumed by Bryde’s whales was higher than that does sei whales.
Discussion
This study reported recent prey consumption, dietary patterns and distribution of baleen whales in relations to environmental factors in a broad research area off the Pacific coast of Japan. Results suggest that this area is an important feeding location for the minke, sei, and Bryde’s whales where they consume large amounts of fish resources near the sea surface that are carried by the Kuroshio Current. Diet overlap between whales also demonstrated how these whales depend on these small pelagic fish in these waters. A bioenergetic mass-balance model has been developed for the western North Pacific, (Mori et al., MS 2009) using Ecopath and Ecosim (Pauly et al., 2000). The consumption estimates of this study contribute that model as input parameters.
What determines the diet of whales in spatio-temporal scale?
Minke whales occurred most commonly in areas with the coldest SST and higher latitude and also in coastal areas. The minke whales tended to feed on large-sized anchovy in May and June, then switch to Pacific saury in July to September. This prey shift was also explained by the factors date, latitude and SST in CCA for diet composition. This clear seasonal change and prey change were observed only in the minke whale. We also found large-sized mackerels in the stomachs of minke whales, however the occurrence was low. This may be related to the occurrence of mackerels under the warm Kuroshio water (above 15°C; Hirai et al., 1988). Small-sized mackerel (0-age) which other whales fed on were also not found in the stomach of minke whales, suggesting minke whales and small sized mackerels were not spatially overlapped. The variation of diet due to their body length was also only observed in the minke whale.
Sei whales occurred at SST from 10 to 24°C which overlaps with most of the occurrences of minke whales, however CCA for whale density could separate the whales in canonical axis 2 explained by chlorophyll a and longitude, suggesting sei whales occurred in offshore waters and areas of lower chlorophyll a concentration.
The variety of prey in sei whale stomachs was lower than that in minke whales, however this species fed on copepods and euphausiids, rather than Pacific saury in addition to Japanese anchovy. The CCA for diet composition showed that anchovy was eaten at warmer SST and western area and copepods were eaten in the opposite environment. 0-aged mackerel was also found in some sei whale stomachs south of 38° N, where minke whales did not occur.
Bryde’s whales occurred at the warmest SST ranging from 16 to 27°C at south of 40° N, showing a clear separation of distribution from the other two whale species which was also explained in CCA for whale density. Bryde’s whales appear to specialize on few prey species in the study area, i.e., Japanese anchovy, euphausiids, mackerels. From the size distribution of anchovy in their stomachs, Bryde’s whales fed on both 0-aged class and adult anchovy. Bryde’s whale fed on euphausiids but not on copepods, which occur in the transition region, suggesting the distribution of this whale is usually within subtropical waters.
CCA for whale density illustrates the characteristics of whale distributions relative to the environmental factors in this study area. This habitat selection regime is the first systematic decision of foraging behaviour for the baleen whales followed by geographical overlapping between whales and their energetically efficient prey, such as pelagic fish or squids. Thus habitat preference information should be combined as a part of foraging study to know real prey selection in ecosystem study in addition to specialized function of prey capture in baleen whales (Berta and Sumich, 1999).
Japanese anchovy, Pacific saury and mackerels occur in the near surface layer (Hatanaka, 1968; Kondo, 1969; Ida, 1972). As many previous diet studies have reported, large balaenopterid species feed on aggregated near surface fish, squids and crustaceans (Nemoto, 1959; Nemoto and Kawamura, 1977; Kawamura, 1982). Although minke whale also feed on variety of prey types including larger fish, such as walleye pollock (Theragra chalcogramma), Japanese pomfret (Brama japonica) and Atlantic cod (Gadus morhua) in addition to the small pelagic fish (Haug, et al., 1995; Lindstrøm, 1998; Tamura and Fujise, 2002), the majority of prey species were small pelagic fish, such as anchovy and saury, throughout the whole study area.
Japanese sardine used to be one of the main prey species for baleen whales captured during the commercial whaling period (Kawamura, 1982; Kasamatsu and Tanaka, 1992), however Japanese sardine is a minor prey in more recent times, as shown in this study. The change in major species composition from sardine to anchovy reported as part of an ecological regime-shift in the 1980s (Yatsu et al., 2001; Takasuka, et al., 2007; Watanabe, 2009) is apparently reflected in the diet of whales, suggesting that these three baleen whale species can modify their diets in response to changes in prey abundance.
Pacific saury, which occur in the superficial layer (Hatanaka, 1968), was one of the main preys for minke and sei whales, and minke whale fed on the large-sized fish of this species. Larger saury (>280 mm) hatched from autumn to winter in the JARPN II survey period (Suyama et al., 1996) are mature, and adult size saury have a higher caloric value compared to other prey, suggesting the minke whales feed on the highly nutritious saury later in the season when mature saury are distributed in the subarctic region.
Since the mackerel consumed by whales were mostly 0-aged fish just carried by Kuroshio current, their consumption by sei and Bryde’s affects the early life stage of this prey, and may have less impact than if they had consumed older fish. This study also showed a greater consumption of mackerels later in the year, suggesting that the mackerel may became more available with years for sei and Bryde’s.
The present study illustrated that environmental variation largely affects the distributions and diets of whales, indicating that their diets largely depend on their preferred “environmental” habitat and that spatio-temporal overlap with preys as a function of the availability and relative abundance of pelagic small fish advected into the study area by the Kuroshio-current extension, with Japanese anchovy being important to all three whale species in recent years. The recruitment, survival and transportation by Kuroshio Current of the anchovy and saury are associated with the change of the Aleutian Low Pressure and weakening of the Oyashio Current in winter (Watanabe, 2009), suggesting that variability of fish populations is also a considerable factor determining the diet of whales in this region.
Prey consumption estimates
This study demonstrated that whale abundance largely affects the total consumption of each prey in addition to the diet composition, i.e., large biomass sei and Bryde’s whales consume overall more anchovy and mackerel than do the minke whales. However consumption estimates are still preliminary with many uncertainties. In this study, we assumed a 150 day feeding period, and that there is a variation in daily consumption amounts because of foraging success and energy contents in prey. An improved understanding of the feeding habits of whales is necessary to adequately deal with these uncertainties and to allow for the development of reliable energy-based ecosystem models. Information of migration patterns through the feeding season and daily feeding behaviour using biotelemetry study will also contribute to reduce the uncertainties.
One conclusion drawn from these comparisons of the commercial catch and the estimated consumption of these three baleen whale species in the area off the Pacific coast of Japan is that these whales may have an important role in relation to their fish prey species within this marine ecosystem. The estimated consumption by whales is similar to that of commercial fisheries for some fish species, therefore it should be factored into fisheries assessments and management regimes.
Acknowledgements
We would like to thank all captains, crews, and scientists, who were involved in the offshore components of JARPN II surveys from 2000 to 2007. Our sincere thanks go to L. A. Pastene and D. Goodman of the Institute of Cetacean Research (ICR) for their valuable suggestions and English corrections on this paper and to A. Tsuda of the University of Tokyo’s Ocean Research Institute for teaching us copepod species identification. Thanks are also due to Yokota-san for her dataset preparation. Finally we thank two anonymous reviewers and editors who gave us good suggestions.
References
BERTA, A., and J. L. SUMICH. 1999. Marine Mammals: Evolutionary biology. Academic Press, USA, 494 p.
FAVORITE, F., A. J. DODIMEAD, and K. NASU. 1976. Oceanography of the Subarctic Pacific region 1960–71. Bull. Int. North Pacific Comm., 33, 1–187.
FIEDLER, P. C., and S. B. REILLY. 1994. Interannual variability of dolphin habitats in the eastern tropical Pacific. II: Effects on abundances estimated from tuna vessel sightings, 1975–1990. Fish. Bull., 92: 451–463.
FISHERIES AGENCY of JAPAN. 2008. Status of fisheries resources around Japan. Fisheries Agency of Japan and Fisheries Research Agency. [In Japanese] http://abchan.job.affrc.go.jp/digests20/index.html
GIRAUDOUX, P. 2008. pgirmess: Data analysis in ecology. R package version 1.3.7. http://perso.orange.fr/giraudoux/SiteGiraudoux.html
GUISAN, A., and N. E. ZIMMERMANN. 2000. Predictive habitat distribution models in ecology. Ecological Modelling., 135: 147–186. doi:10.1016/S0304-3800(00)00354-9
HAUG, T., H. GJØÆSTER, U. LINDSTRØM, and K. T. NILSSEN. 1995. Diet and food availability for north-east Atlantic minke whales (Balaenoptera acutorostrata), during the summer of 1992. ICES J. Mar. Sci., 52: 77–86.
HAKAMADA, T., K. MATSUOKA, and T. MIYASHITA. MS 2009. Distribution and the number of western North Pacific common minke, Bryde’s, sei and sperm whales distributed in JARPN II Offshore component survey area. Paper SC/J09/JR15 presented to the JARPN II Review Workshop, Tokyo, January 2009 (unpublished, http://www.iwcoffice.org/_documents/sci_com/workshops/SC-J09-JRdoc/SC-J09-JR15.pdf - link n/a), 12 p.
HATANAKA, M. 1956. Biological studies on the population of the saury, Cololabis saira (brevoort) Part 2 Habits and Migrations. Tohoku J. Agric. Res., 6: 313–340. http://ci.nii.ac.jp/vol_issue/nels/AA0086391X/ISS0000099568_en.html
HATANAKA, H., and T. MIYASHITA. 1997. On the feeding migration of the Okhotsk Sea-West Paciric stock of minke whales, estimates based on length composition data. Rep. Int. Whal. Comm., 47: 557–564.
HIRAI, H., Y. OGAWA, K. OKUDA, I. YASUDA, and H.YAMAGUCHI. 1988. Fluctuation of hydrographic conditions in relation to the formation, movement and disappearance of Purse Seine fishing grounds of cuhb mackerel in waters off Sanriku, Northeastern Japan. Bull. Tohoku Reg. Fish. Res. Lab., 50: 133–151. [In Japanese with English abstract]
HOSOKAWA, H., and T. KAMIYA. 1971. Some observations on the cetacean stomachs, with special considerations on the feeding habits of whales. The Scientific Reports of the Whales Research Institute, 23: 91–101.
IDA, H. 1972. Some ecological aspects of larval fishes in waters off central Japan. Bull. Jpn. Soc. Sci. Fish., 38: 981–994.
IWC. 1994. Report of the Working Group on North Pacific minke whale management trials. Rep. Int. Whal. Comm., 44: 120–144.
ITOH, S., and S. KIMURA. 2007. Transport and survival of larvae of pelagic fishes in Kuroshio system region estimated with Lagrangian drifters. Fish. Sci., 73: 1295–1308.
KASAI, H., H. SAITO, A. YOSHIMORI, and S. TAGUCHI. 1997. Variability in timing and magnitude of spring bloom in the Oyashio region, the western subarctic Pacific off Hokkaido, Japan. Fish. Oceanogr., 6:118–129. doi:10.1046/j.1365-2419.1997.00034.x
KASAMATSU, F., and S. TANAKA. 1992. Annual changes in prey species of minke whales taken off Japan 1948–87. Nippon Suisan Gakk., 58: 637–651.
KAWAMURA, A. 1982. Food habits and prey distributions of three rorqual species in the North Pacific. Sci. Rep. Whales Res. Inst., 34: 59–91.
KONDO, K. 1969. Ecological studies of life pattern of the Japanese anchovy, Engraulis japonicus (HOUTTUYN). Bulletin Tokai Region Fishery Research Laboratory, 60: 29–81.
LEAPER, R., and D. LAVIGNE. 2007. How much do large whales eat? J. Cetacean Res. Manag., 9: 179–188.
LINDSTRØM, U., Y. FUJISE, T. HAUG, and T. TAMURA. 1998. Feeding habits of Western North Pacific minke whales, Balaenoptera acutorostarta, as observed in July–September 1996. Rep. Int. Whal. Comm., 48: 463-469.
LOCKYER, C., 1981. Growth and energy budgets of large baleen whales from the Southern Hemisphere. In: Mammals in the Sea. FAO Fish. Ser, No. 5, 3: 379–487.
1987. The relationship between body fat, food resource and reproductive energy costs in north Atlantic fin whales (Balaenoptera physalus). Symp. Zool. Soc. Lond., 57: 343–361.
MASAKI, Y. 1976. Biological studies on the North Pacific sei whale. Bull. Far Seas Fish. Res. Lab., 14: 1–103.
MORI, M. MS 2009. Development of an ecosystem model of the western North Pacific. Paper SC/J09/JR21 presented to the JARPN II Review Workshop, Tokyo, January 2009 (unpublished, http://www.iwcoffice.org/_documents/ sci_com/workshops/SC-J09-JRdoc/SC-J09-JR21.pdf - link n/a), 12 p.
NEMOTO, T. 1959. Food of baleen whales with reference to whale movements. Sci. Rep. Whales Res. Inst., 14: 149–290.
NEMOTO, T., and A. KAWAMURA. 1977. Characteristics of food habits and distribution of baleen whales with special reference to the abundance of North Pacific sei and Bryde’s whales. Rep. Int. Whal.Comm., Special issue 1: 80–87.
OLSON, D. B. 2001. Biophsical dynamics of western transition zones: a preliminary synthesis. Fish. Oceanogr., 10: 133–150. doi:10.1046/j.1365-2419.2001.00161.x
OLSEN, M. A., E. S. NORDØY, A. S. BLIX, and S. D. MATHIESEN. 1994. Functional anatomy of the gastrointestinal system of Northeastern Atlantic minke whales (Balaenoptera acutorostrata). J. Zool., 234: 55–74. doi:10.1111/j.1469-7998.1994.tb06056.x
OKSANEN, J., K. ROELAND, P. LEGENDRE, B. O'HARA, G. L. SIMPSON, M. HENRY, H. STEVENS, and H. WAGNER. 2008. vegan:Community Ecology Package. R package version 1.13-1. http://vegan.r-forge.r-project.org/
PAULY, D. L., A. W. TRITES, E. CAPULI, and V. CHRISTENSEN. 2000. Ecopath, Ecosim, and Ecospace as tools for evaluating ecosystem impact of fisheries. ICES J. Mar. Sci., 55: 467–481.
PIANKA, E. R. 1973. The structure of lizard communities. Annu. Rev. Ecol. Syst., 4: 53–74. doi:10.1146/annurev.es.04.110173.000413
1974. Niche overlap and diffuse competition. Proc. Nat. Acad. Sci. USA., 71: 2141–2145. doi:10.1073/pnas.71.5.2141
REDFERN, J. V., M. C. FERGUSON, E. A. BECKER, K. D. HYRENBACH, C. GOOD, J. BARLOW, K. KASCHNER, M. F. BAUMGARTNER, K. A. FORNEY, L. T. BALLANCE, P. FAUCHALD, P. HALPIN, T.
HAMAZAKI, A. J. PERSHING, S. S. QIAN, A. READ, S. B. REILLY, L. TORRES, and F. WERNER. 2006. Techniques for cetacean-habitat modeling. Mar. Ecol. Prog. Ser., 310: 271–295.
REILLY, S. B., and P. C. FIEDLER. 1994. Interannual variability of dolphin habitats in the eastern tropical Pacific. I:Research vessel surveys, 1986–1990. Fish. Bull., 92: 434–450.
SIGURJÓNSSON, J., and G. A. VÍKINGSSON. 1997. Seasonal abundance of and estimated prey consumption by cetaceans in Icelandic and adjacent waters. J. Northw. Atl. Fish. Sci., 22: 271–287. doi:10.2960/J.v22.a20
SHIOMOTO, A., K. SASAKI, T. SHIMODA, and S. MATSUMURA. 1994. Primary productivity in the offshore Oyashio in the spring and summer 1990. J. Oceanogr., 50: 209–222. doi:10.1007/BF02253480
SUYAMA, S., Y. SAKURAI, and K. SHIMAZAKI. 1996. Age and growth of Pacific saury Cololabis saira (Brevoort) in the Western North Pacific Ocean estimated from daily otolith growth increments. Fish. Sci., 62: 1–7.
TAKAHASHI, M., Y. WATANABE, T. KINOSHITA, and C. WATANABE. 2001. Growth of larval and early juvenile Japanese anchovy, Engraulis japonicus, in the Kuroshio-Oyashio transition region. Fish. Oceanogr., 10: 235–247. doi:10.1046/j.1365-2419.2001.00160.x
TAKAHASHI, M., and Y. WATANABE. 2004. Growth rate-dependent recruitment of Japanese anchovy Engraulis japonicus in the Kuroshio-Oyashio transitional waters. Mar. Ecol. Prog. Ser., 266: 227–238. doi:10.3354/meps266227
TAKASUKA, A., Y. OOZEKI, and I. AOKI. 2007. Optimal growth temperature hypothesis: Why do anchovy flourish and sardine collapse or vice versa under the same ocean regime? Can. J. Fish Aquat. Sci., 64 768–776. doi:10.1139/F07-052
TAMURA T., Y. FUJISE, and K. SHIMAZAKI. 1998. Diet of minke whales Balaenoptera acutorostrata in the North western part of the North Pacific in Summer, 1994 and 1995. Fish. Sci., 64: 71–76.
TAMURA, T., and Y. FUJISE. 2002. Geographical and seasonal changes of prey species of minke whale in the Northwestern Pacific. ICES J. Mar. Sci., 59: 516–528.
TRITES, A., V. CHRISTENSEN, and D. PAULY. 1997. Competition between fisheries and marine mammals for prey and primary production in the Pacific Ocean. J. Northw. Atl. Fish. Sci., 22: 173–187. doi:10.2960/J.v22.a14
VÍKINGSSON, G. A. 1995. Body condition of fin whales during summer off Iceland. In: Whales, seals, fish and man. A.S. Blix, L. Walløe and O. Ulltang (eds.). Elsevier, Amsterdam, p. 361–369.
WATANABE, Y. 2009. Recruitment variability of small pelagic fish populations in the Kuroshio-Oyashio transition region of the Western North Pacific. J. Northw. Atl. Fish. Sci., 41: 197–204.
YATSU, A., H. NISHIDA, M. ISHIDA, A. TOMOSADA, T. KINOSHITA, and T. WADA. 2001. Trajectories of catch and stock abundance of dominant small pelagic fishes and common squid with some related environmental indices around Japan. PICES Sci. Rep., 18: 175–178.
|