Introduction
The Canadian Department of Fisheries and Oceans (DFO) conducted a series of mesopelagic fish surveys in 1984–89 in an area that extended from about 50°W, south of Grand Bank, to about 64°W, off the western Scotian Shelf (Fig. 1). This area is the north-eastern part of an oceanographic water body, named the Slope Sea by Csanady and Hamilton (1988), that extends to Cape Hatteras in the southwest. Its northern edge is the continental slope and its southern edge the Gulf Stream (GS). It is occupied in the southwest by Warm Slope Water (WSW) and in the northeast by cold Labrador Slope Water (LSW), the location of the boundary between these varying substantially depending on the volume of cold water transported around the tail of Grand Bank.
Backus et al. (1977), in a review of Atlantic mesopelagic zoogeography, recognized WSW as the most westerly province in a North Atlantic temperate region, the adjacent LSW being the most southwesterly component of a subarctic region to the northeast. Thus, the DFO surveys were designed to include sampling locations in both WSW and LSW.
|
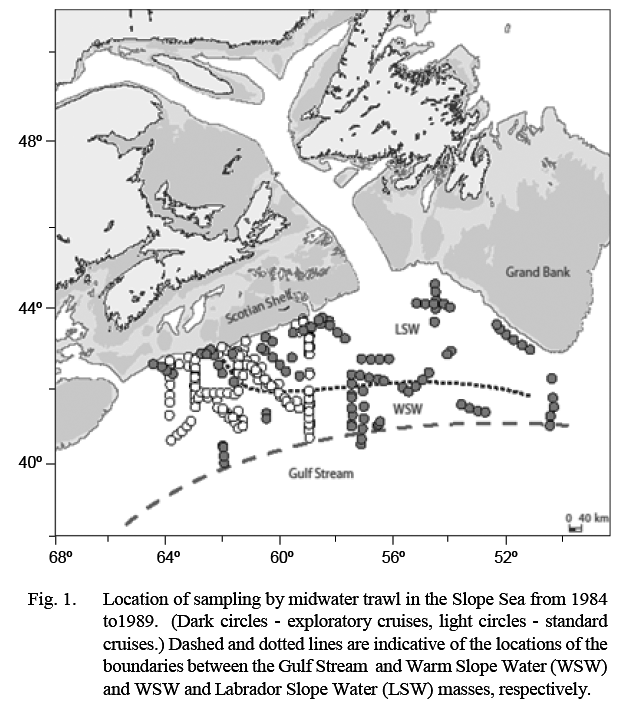 Fig. 1 Fig. 1
|
An inventory of the mesopelagic fishes caught during the DFO surveys (Themelis and Halliday, 2012) identified the myctophids Benthosema glaciale (Reinhardt, 1837) (the glacier lanternfish) and Ceratoscopelus maderensis (Lowe, 1839) (the horned lanternfish) as the dominant species in catches in LSW and WSW respectively. The present paper examines and compares the biology of these two species in these water masses, to provide a fuller understanding of their status in each area and thus improve knowledge of the biogeography of the Slope Sea. Biological features examined are distribution, growth, reproduction and diet. Larval distributions over the continental slope are described also based on data from ichthyoplankton surveys conducted by DFO (O’Boyle et al., 1984) and the USA National Marine Fisheries Service (NMFS) (Morse et al., 1987).
Methods
Ten surveys were conducted in the period 1984–89. The number of tows conducted in each water mass on each survey is listed in Table 1. Listings of station coordinates and maps are available in Halliday et al. (1995). The first four surveys were exploratory, and areas fished extended variously from about 50°–65°W and from the shelf edge to 40°N (Fig. 1). The subsequent six standardized cruises were conducted between February 1988 and August 1989, arranged temporally to provide sampling every second calendar month and restricted spatially to 59°–64°W and from the shelf edge to about 41°N. Sampling on these standardized surveys was conducted along three north-south transects at 59°, 61°30' and 64°W linked by diagonal transects. This sampling block was chosen because the exploratory cruises had shown that it typically contained both LSW and WSW.
|
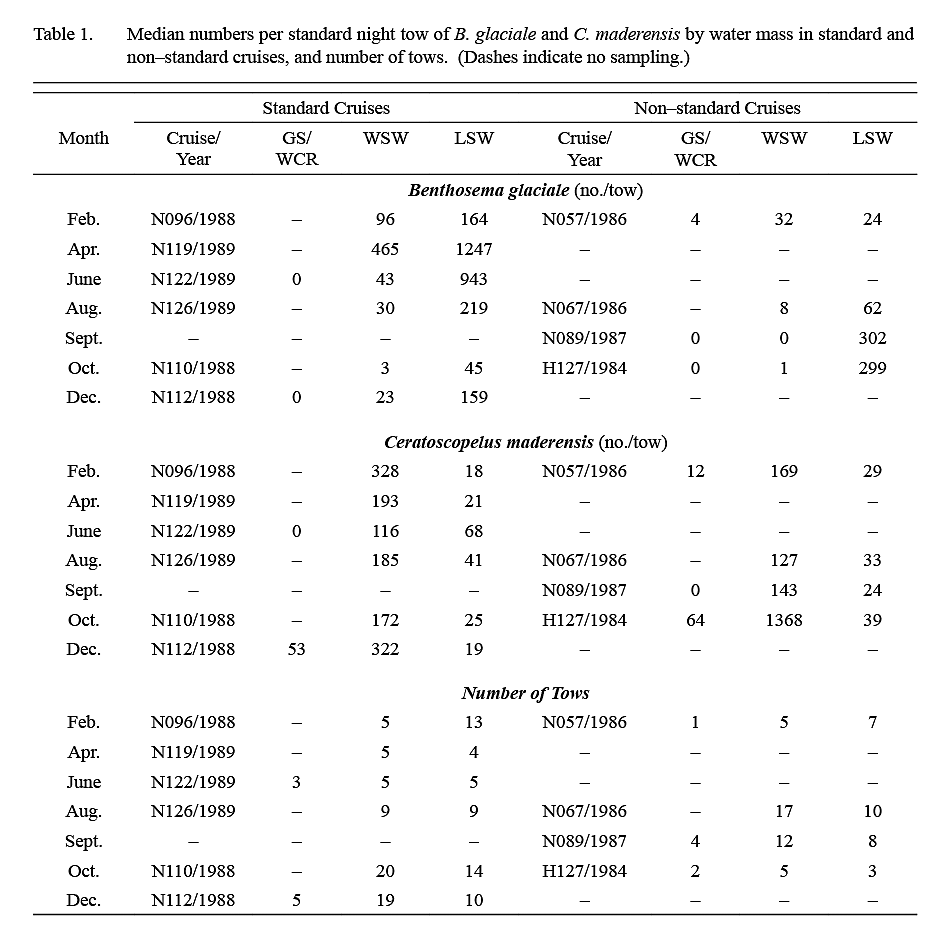 Table 1 Table 1
|
The sampling gear used was an International Young Gadoid Pelagic Trawl (IYGPT) (Hislop, 1970), a twin-warp mid-water trawl with an 11.5 m (horizontal) by 8.5 m (vertical) mouth opening (measured using a SCANMAR net management system). A three-step oblique night tow, with the net towed for 10 min. at 200, 100 and 50 m, was adopted as a standard deployment. Night was defined as extending from one hour after sunset to one hour before sunrise. Total fishing time including haul back was about 40 min. On the first survey (H127) the third step was at about 20 m and on the second survey (N057) depth monitoring gear failed and continuous oblique tows from 200-300 m to the surface were conducted, but the results from these are accepted as comparable to those from subsequent standard tows.
Other, non-standard, IYGPT tows were made opportunistically during the day and those made at depths of 350–1000 m are used here to describe deep water daytime catches. Only those tows made at locations where bottom depth was greater than 1000 m are used to avoid shelf edge effects on catches.
Profiles of water temperatures from the surface to at least 460 m (and sometimes as deep as 1830 m) were collected at all fishing stations, and at intermediate locations. A description of oceanographic equipment and methodologies used, station distributions, and an analysis of the hydrographic data collected, are provided by Halliday et al. (1995).
Previous studies of mid-water fishes in the Northwest Atlantic have defined water masses by temperatures at 200 m as follows: LSW<9°C; 9°C ≥ WSW <15°C; GS≥ 15°C; (after Worthington, 1964), and these criteria were used here to classify fishing stations by water mass in summer surveys. However, in winter, it was found that ‘transition areas’ between water masses occurred when WSW was overlain by a cold upper layer. Tows in such transition areas were combined with those clearly in WSW in October and December surveys and with those clearly in LSW in February, based on overall faunal similarities (Themelis, MS 1996).
All fishes were separated from catches at sea and preserved in 10% formalin. On subsequent examination ashore, these were identified to species, categorized as larvae (pre-metamorphic) or adults (post-metamorphic) and, within each of these categories, the number caught and their minimum and maximum sizes ((standard length - SL) to the nearest mm)) were recorded. Specimens were then transferred to 50% isopropanol for long-term preservation and archived at the Atlantic Reference Centre, Huntsman Marine Science Centre, St. Andrews, N.B., Canada.
The metric chosen to describe relative abundance (density) was the median of catch numbers per tow, as numbers per tow were not normally distributed and zero catches occurred regularly, making both arithmetic and geometric means unsuitable.
Subsets of the catches of B. glaciale and C. maderensis from each of the six standard cruises in 1998–99 were arbitrarily selected from each water mass for examination in detail. From each selected sample, up to 300 fish were measured for standard length (larger catches being randomly sub-sampled). From these, length-stratified subsamples were examined for sex, maturity, fecundity and stomach contents.
The sexual development of females of both species was classified into the stages immature, ripening (occurrence of visible eggs), ripe (occurrence of some hydrated eggs), spent (occurrence of residual eggs), or resting. Maturity ogives were calculated as the ratio of numbers at ripening, ripe and spent stages to the total at all stages, by length group, during the defined spawning season. The ‘total at all stages’ category included half of those specimens too small for sex to be determined (typically fish <25 mm). In contrast to females, the reproductive organs of males did not exhibit features that provided an objective basis for estimating size at maturation. In B. glaciale, however, the state of development of luminous caudal glands (a single supracaudal gland on males and a pair of smaller infracaudal glands on females) was noted, as the occurrence of these has been associated with sexual maturation (Gjøsæter, 1981; Kawaguchi and Mauchline, 1982).
Fecundity estimates were obtained by counting all maturing eggs in the ovaries of those female individuals classed as “ripe”, i.e. those in which some hydrated eggs were observed.
Stomach fullness was assessed visually as empty (coded 0), containing a small amount of food (1), about ½ full (2), full (3) or distended (4), following Gjøsæter (1973). The proportion of the contents (by volume) that could not be assigned to a taxonomic group because of digestion was classified as nil (0), less than ¼ (1), ¼ to ½ (2), ½ to ¾ (3), ¾ to <1 (4) and all (5).
Information on larval distributions was extracted from the data archives for the ichthyoplankton surveys conducted over the Scotian Shelf by DFO in 1976–82 (O’Boyle, et al., 1984) and over shelf waters adjacent to the USA coast by NMFS in 1977–87 (Morse et al., 1987). Both survey series extended seaward into continental slope waters and sampling was distributed over all months of the year. Catch numbers of B. glaciale and C. maderensis were summed by month and by longitude to provide an account of temporal and spatial distribution of catches. No corrections were made to account for differences in fishing effort among months or areas.
Results
Benthosema glaciale
Distribution
The median catch per tow of B. glaciale in IYGPT standard night tows (Table 1) was higher in LSW than it was in WSW in all but the first exploratory survey. Among the six standard surveys, the lowest differentials between water masses were observed in samples from February and April (ratio of LSW/WSW = ~x2 and ~x3 respectively) whereas for the other four surveys this ratio was in the range x7–x22. The median of the ratios for all six standard surveys was x7. However, in the deep day tows conducted during standard surveys, the differential in density between LSW and WSW was about x3 in both winter (December–April) and summer (June–October) (Table 2).
|
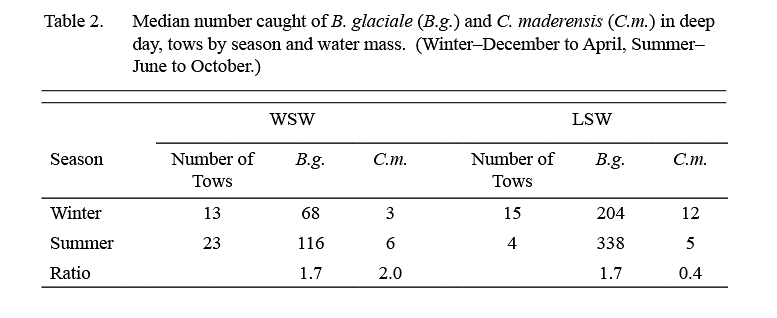 Table 2 Table 2
|
Length compositions by water mass
The length compositions of B. glaciale caught in standard night tows on the six standard cruises in 1998-99 were closely similar in shape in LSW and WSW (Fig. 2). Although fewer small fish (20–30 mm) occurred in WSW than in LSW in February 1988 samples, this was likely due to sampling variation as this mode was of comparable prominence in both water masses in the length compositions from other months. A recruitment event was indicated by the occurrence in August samples of a strong mode at about 18 mm (range: 13–25 mm). These post-metamorphic age 0 fish formed the dominant mode also in October and December samples, becoming progressively less prominent in subsequent months but traceable to the mode at 37 mm, again in August samples, at age 1. Larger modes, representing older age groups, were also present, and fish up to a length of about 65 mm were not uncommon. In standard night tows overall, the largest specimen recorded was 71 mm.
Catches of B. glaciale in deep day tows were composed of larger fish than those caught in standard night tows. The minimum and maximum lengths in catches made in deep day tows ranged from 18–79 mm, compared to 13–71 mm in night tows. The median of minimum lengths was 31mm, compared to 25 mm in standard night tows, indicating that the smallest post-metamorphic animals were not available to the day tows. The median of maximum lengths was 65 mm, compared to 57.5 mm in standard night tows, indicating that the largest fish in the population were not fully represented in the shallow standard night tows. These differences are illustrated by the length composition measurements from the October 1988 (N110) survey as, in that survey, standard night and deep day samples were collected in both WSW and LSW (Fig. 3).
|
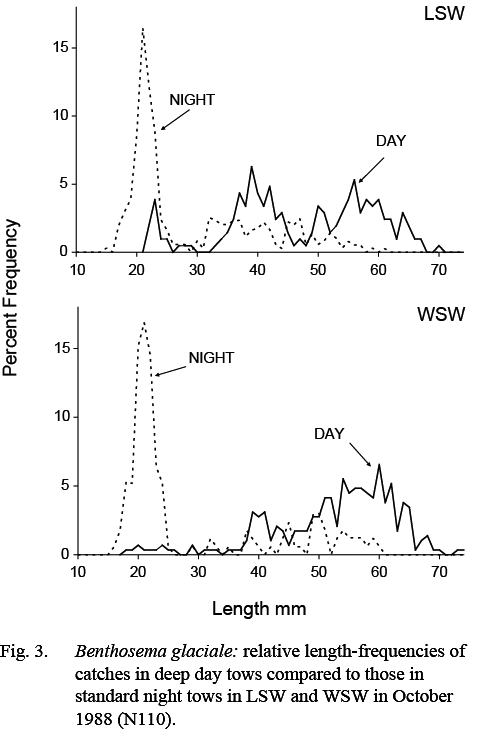 Fig. 3 Fig. 3
|
Larval occurrences
About 1,000 larval B. glaciale (defined as animals <12 mm) were caught in ichthyoplankton surveys conducted by DFO in 1976–82 and about 13,000 larvae were caught in 1977–87 NMFS surveys. Catches occurred throughout the areas sampled (57°W-67°W by DFO, 67°W to 75°W by NMFS (Table 3)), at stations located over or adjacent to the continental slope. In both survey series, 98% of catches occurred in April–June (Table 3).
|
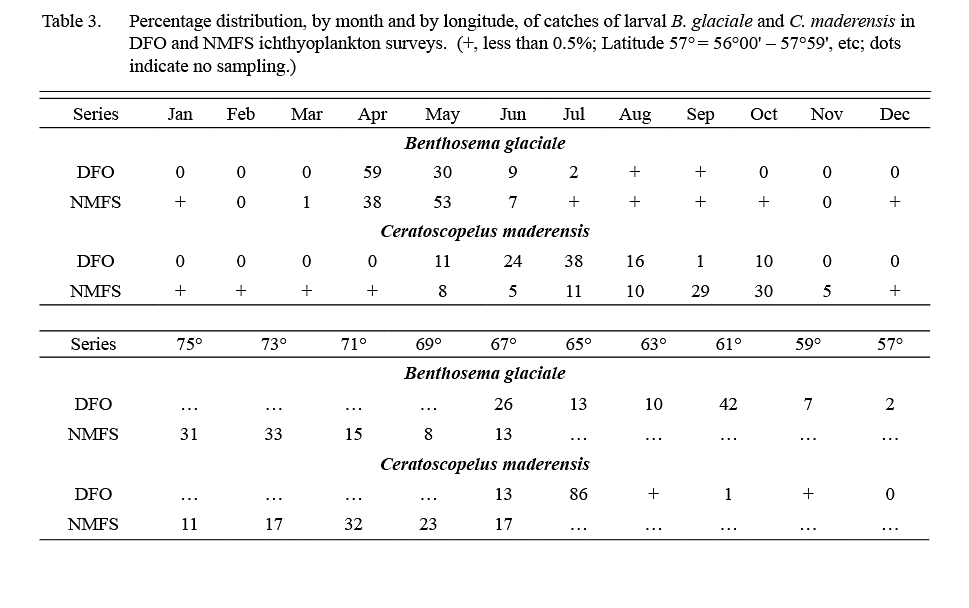 Table 3 Table 3
|
Reproduction
Females categorized as ripe, i.e. ovaries contained some translucent eggs, occurred in October, December, February and April catches, although primarily in December–February. Spent fish were noted in samples from December to June. This pattern of temporal distribution in occurrences of ripe females was noted in samples from both LSW and WSW, indicating a general coincidence in the timing and duration of spawning between water masses.
The maturity stage data from December, February and April samples were chosen for calculation of length at sexual maturation. The resulting maturity ogives for females differed between water masses (Fig. 4). Length at 50% maturity (L50) was 32–33 mm in WSW (length range over which maturation occurred was approximately 20–45 mm) and L50 was about 39-40 mm in LSW (maturation length range was approximately 30–55 mm).
|
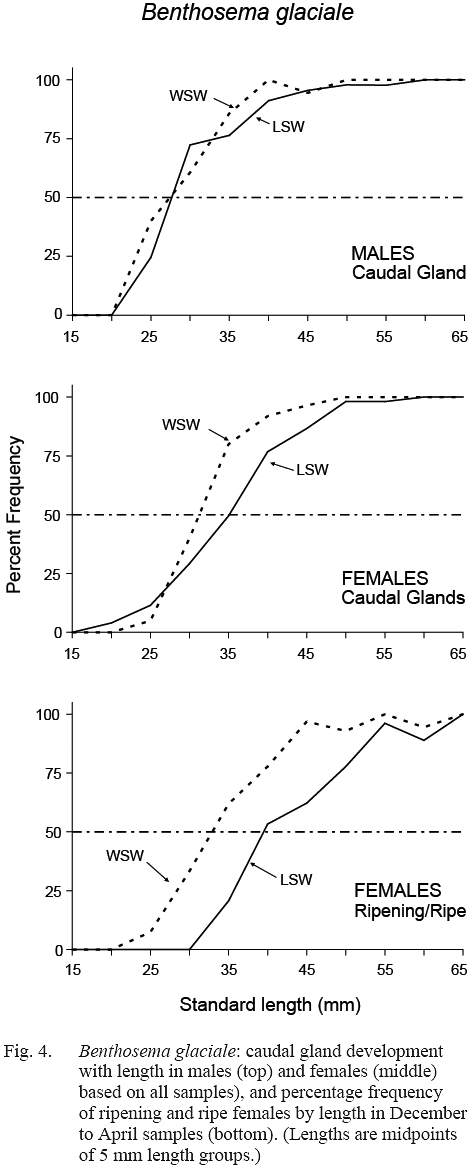 Fig. 4 Fig. 4
|
The ratio of females exhibiting full infracaudal gland development versus those with no, or partial development, when plotted against length, gave ogives which also differed by water mass, L50 being 31 mm in WSW and 35 mm in LSW (Fig. 4). This is in general agreement with the result of direct gonad maturity staging, maturation preceding egg development by a few millimetres in fish length. In males, gland development did not differ between water masses, L50 occurring at 27–28 mm in both cases, suggesting that males mature at a smaller size than females.
A count was obtained of the total number of eggs in the ovaries of each of 97 ripe females. These specimens, which ranged in length from 26 to 70 mm, came predominantly from December and February samples, with a few from October (3) and April (10), and most (74%) came from stations classed as being in WSW. Fecundity averaged 583 eggs (range = 140–1098) and was significantly correlated with fish length (r = 0.633, df = 95, p <0.01).
Feeding and food composition
Stomach fullness of B. glaciale varied seasonally (Fig. 5), being highest in summer (more than ½ full) and lowest in winter-spring, particularly in April when stomachs were close to empty. Much of the food in the stomachs was unidentifiable in all months but more so in February–June.
|
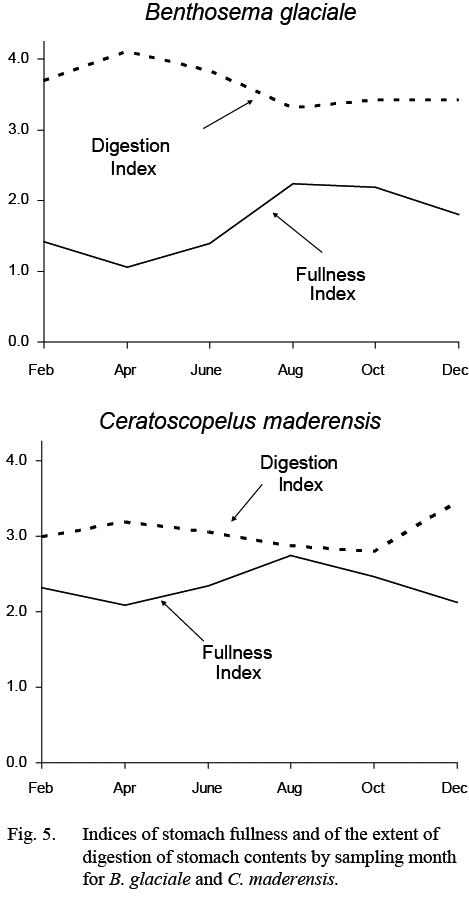 Fig. 5 Fig. 5
|
Calanoid copepods comprised almost 75% of identifiable dietary items (Table 4), followed in importance by euphausids (15–20%). Food items were of roughly similar importance in February–June ‘winter’ and August–December ‘summer’ samples except for a higher importance of Pleuromamma spp. in summer.
Ceratoscopelus maderensis
Distribution
The median catch per tow of C. maderensis was higher in WSW than it was in LSW in all surveys (Table 1). However, the differential in density between water masses varied seasonally, being about x17 higher in WSW in winter and about x4 higher in summer (Table 2). Very few C. maderensis were caught in deep day tows in either season (Table 2).
|
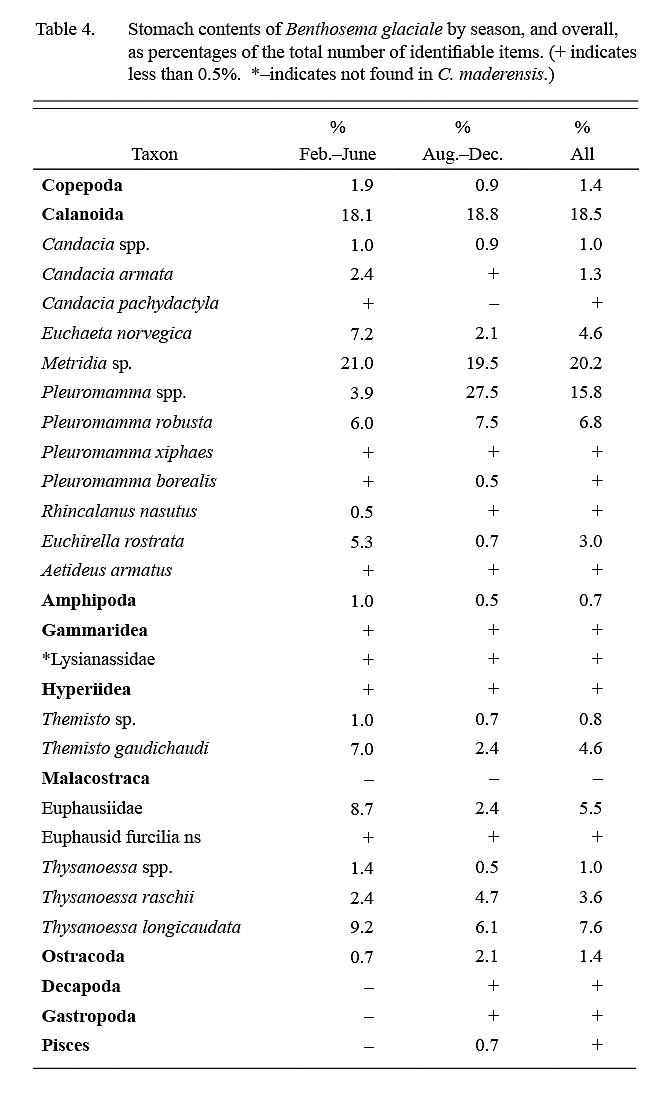 Table 4 Table 4
|
Length compositions by water mass
The length compositions of catches in standard IYGPT night tows differed substantially between water masses. Catches in LSW were composed predominantly of large fish and those from WSW of small fish (Fig. 6).
In WSW, the August catch was composed almost exclusively of larval and immediately post-metamorphic fish with modes of 13 and 19 mm, respectively. These recruits can be followed in the length-frequencies of October and December catches as a multi-modal group extending from 15 to 40 mm. The multi-modality of the lengths suggests that there were several waves of spawning. By February, these groups had coalesced into a single mode at 35mm (range 25–45 mm), and this mode was observable at about 48 mm in April and June samples (ranges 35–55 mm and 40–60 mm, respectively). Although missing in August samples, this mode was observed in October catches (at 45–60 mm), but was absent thereafter, indicating a lifespan of one year in WSW.
|
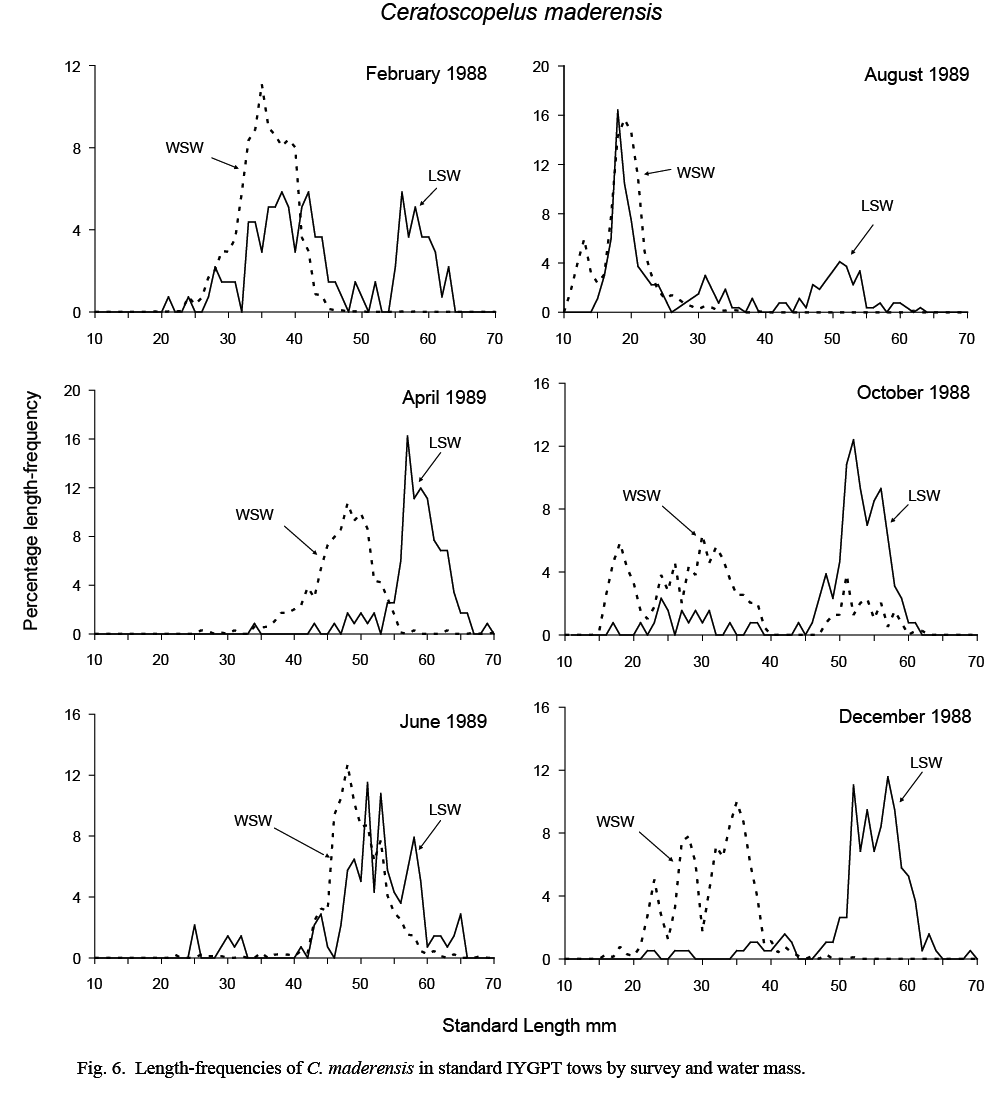 Fig. 6 Fig. 6
|
Catches from LSW were dominated by fish larger than about 45 mm. Although recruiting post-metamorphic fish formed a substantial proportion of catch numbers in August, few were caught in October and none in December. Some fish of this age group reoccurred at 30–45 mm in February samples, but not in April. The February occurrence was likely an assignment error caused by the difficulties in assigning water mass to tows made in the large transition area that existed in that month. The mode in the length frequency of large fish at 45–55 mm in August samples progressed in subsequent months to about 55–65 mm in April. However, in June, this modal length group was smaller, at 45–60 mm, and overlapped that in WSW, suggesting a transfer of young-of-the-year fish from WSW to LSW at this time. It is in this month also that catch rates were highest in LSW (Table 1), supporting such a conclusion.
The length ranges of the C. maderensis caught in deep IYGPT day tows reflected the dichotomy in the length distributions observed in standard night catches. The medians of the minimum and maximum lengths in WSW day tows were 29 mm and 38 mm but, in LSW day tows, were 55 mm and 59 mm. The largest specimen recorded overall was 75 mm. It was caught in a standard night tow in WSW during exploratory cruise N067 in August 1986.
Larval occurrences
The DFO ichthyoplankton surveys caught about 2000, and NMFS surveys caught about 43 000 C. maderensis larvae (animals <18 mm), predominantly over the continental slope. Almost all the larvae caught in DFO surveys were from stations at 64–68°W, i.e. at the most western part of the DFO sampling area (Table 3). In NMFS surveys, however, catches of larvae occurred in abundance from Georges Bank to Cape Hatteras (Table 3). Larvae were recorded from May to October in DFO surveys whereas in NMFS surveys substantial quantities were caught also in November and a few were recorded in December–April, indicating some spawning year-round off the USA coast (Table 3).
Reproduction
The spawning season, as defined by the occurrence in samples of females with ripe eggs, extended from April to October, such occurrences being most frequent in June and August samples. This gonad development occurred in specimens from LSW as well as in those from WSW.
Fish with gonads sufficiently developed for sex to be determined were first noted at about 25 mm in length and essentially all fish larger than 35 mm could be sexed. The smallest female classified as ripening was 44 mm, but most in ripening/ripe/spent stages were greater than 55 mm (Fig. 7). The maturity ogive for females, based on April–August data, was similar in LSW and WSW (Fig. 7). (As noted previously, no inferences could be made regarding size at maturity of males.)
|
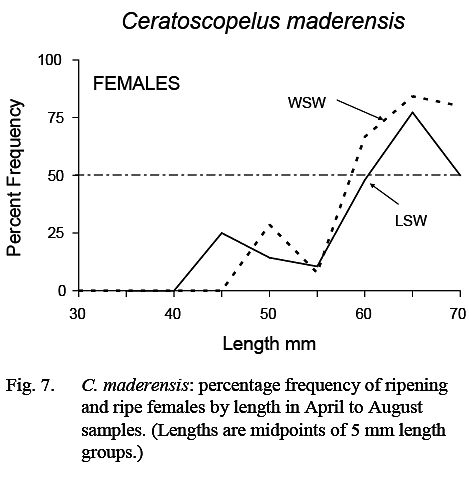 Fig. 7 Fig. 7
|
A count was obtained of the total number of eggs in the ovaries of each of 33 ripe females. These specimens, which ranged in length from 58 to 70 mm, came predominantly from June and August samples, with a few from April (2) and October (5). Sampling was divided equally between WSW (17 fish) and LSW (16 fish). Fecundity averaged 5569 eggs (range = 2134–9632). Fish length explained only 14% of this variation (r = 0.368, df = 31, p <0.05).
The prolonged spawning season, the fact that all fish large enough to spawn do not do so at the same time (Fig. 7), and the lack of correlation between number of eggs and fish length, indicate that individual fish spawn several times during the spawning season.
Feeding and food composition
Stomach fullness was greater than 50% (index >2.0) in all months. There was, nonetheless, some evidence for a maximum in fullness in August and a minimum in April and for a coincident inverse trend in the extent to which the contents were digested (Fig. 5).
Calanoid copepods were the most important dietary contributors overall, comprising about 50% of specimens observed, amphipods, euphausids and chaetognaths accounting, almost equally, for most of the remainder (Table 5). Most food items were of importance in both winter and summer except that chaetognaths contributed to diet mainly in winter (Feb.–June) and gastropods (likely pteropods) mainly in summer (Aug.–Dec.).
|
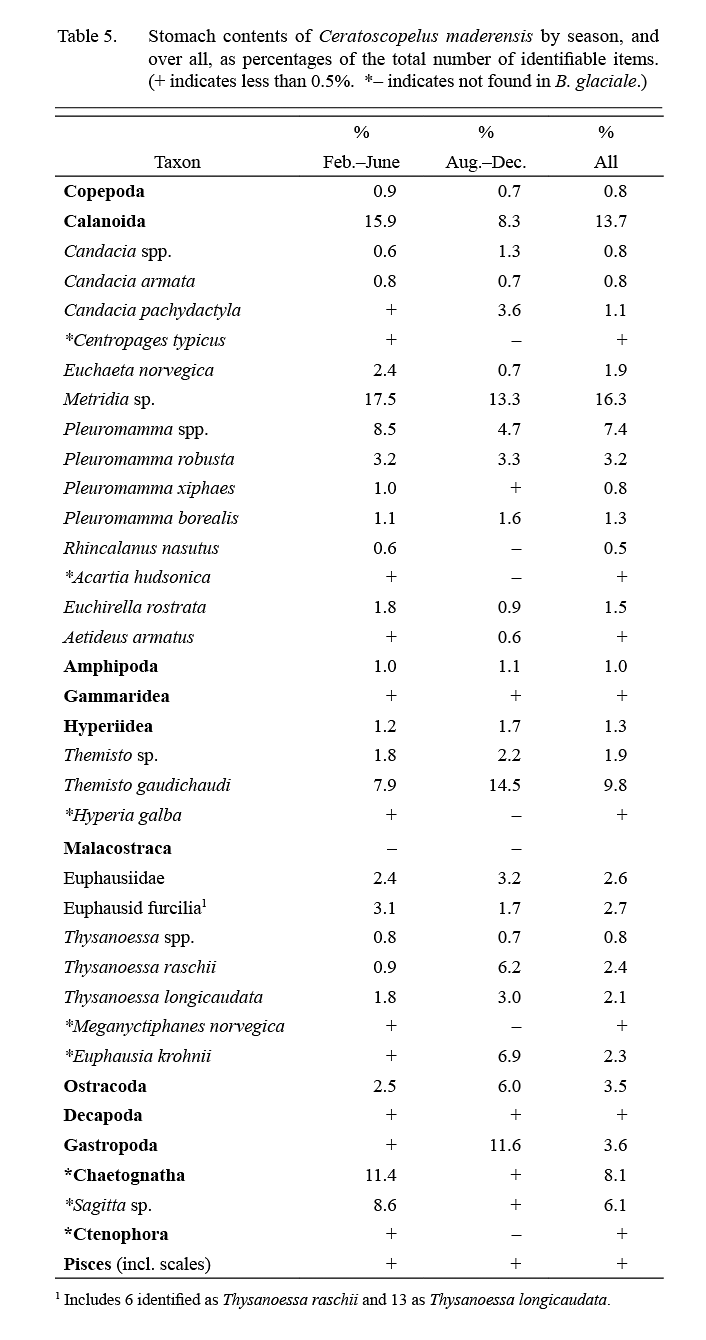 Table 5 Table 5
|
Discussion
Benthosema glaciale
Benthosema glaciale has a North Atlantic sub-polar – temperate distribution (Backus et al., 1977). In the Western Atlantic, it has been recorded as far north as the Davis Strait (Sameoto, 1989) and accounted for about 95% of the myctophid specimens in the collections of Backus et al. (1977) and McKelvie (1985a) from subarctic waters east of Grand Bank. McKelvie (1985b) found that B. glaciale predominated also in mesopelagic fish samples from mixed water masses southwest of Grand Bank, i.e. in the northeastern part of the Slope Sea. Based on present samples, Themelis and Halliday (2012) confirmed the predominance of this species in the LSW that occupies much of the northeastern portion of the Slope Sea, but found that the species ranked only third in abundance in catches made southwest of the LSW/WSW boundary. The species distribution in the eastern Atlantic similarly extends from arctic to temperate waters, including the Mediterranean Sea (e.g. Olivar et al., 2012).
The present analysis shows that the density of B. glaciale was substantially higher in LSW than in WSW in all seasons, based on standard night tows. The lower differentials in density between water masses observed in samples from February and April, compared to other months, may indicate seasonal differences, but the number of samples was small and the differences may be sampling anomalies. Catch rates in deep day tows also were higher in LSW than in WSW, but the difference between median catches was less, x3 on average, regardless of season. There was a difference also in the size compositions of the populations caught at different depths, catches in deep day tows being predominated by large animals, greater than 30mm, in contrast to shallow night tows, in which animals smaller than that, particularly those that were young of the year, were prominent.
Samples from standard cruises reflect conditions in the general proximity of the boundary between LSW and WSW and thus present data may minimize the differences in abundance between the water masses overall. The earlier exploratory cruises, which included samples from more eastern areas (Fig. 1), showed higher differentials in catch rates in LSW vs. WSW. No sampling was conducted during the present study in areas to the west of the standard sampling area. However, Backus and Craddock (1977) recorded high catches of B. glaciale in more western parts of WSW also, suggesting that present data can be taken as indicative of WSW more generally. Gartner et al. (2008) reported captures of adults at the southernmost extent of WSW off Cape Hatteras (about 35°N), and the captures of larval stages along the entire continental slope to Cape Hatteras, indicate that B. glaciale is capable of completing its life cycle throughout WSW as well as in LSW. Transport of specimens of B. glaciale into semi-subtropical waters south of the Gulf Stream in cold-core rings has been reported but available evidence indicates that the species does not reproduce there (Backus and Craddock, 1982; Karnella, 1987).
The size composition of catches was similar in WSW and LSW, suggesting that annual recruitment success and subsequent growth rates were similar in the two areas. A lifespan of 4–5 years was determined, using otolith ring counts, by Halliday (1970) for fish caught over the Nova Scotia continental slope. It appears, however, that the population sampled by Halliday (1970) did not include the full size/age range of the Slope Sea population. His largest specimen had a standard length of 67 mm, whereas Sameoto (1988) subsequently reported an 83 mm specimen from the same slope area and fish up to 79 mm were caught in present samples collected over adjacent oceanic depths. Gjøsæter (1981) reported captures of this species in Norwegian waters that were in the 70–80 mm length range and as old as 7–8 years, and present data indicate that similar lengths and ages are attained off Nova Scotia. The largest length recorded for the species is 98.5mm SL (estimated total length: 103 mm) for a specimen caught off Norway and estimated by Gjøsæter (1973) to be 7–8 years old.
In both water masses, spawning occurred in winter and larvae were most abundant in the plankton in April–May, at least along the continental slope. Post-metamorphic fish were first caught in the IYGPT net in August at about 18 mm and lengths progressed to about 20–30 mm by the following winter at age 1. However, there were differences between water masses in size at maturity, females in WSW maturing at a smaller size than those in LSW. In WSW, the maturity ogive included fish that were in the 20–30 mm size range, indicating that some age 1 and most age 2 fish matured. In LSW, however, essentially no age 1 females, and less than half of age 2 females were large enough to spawn, as found to be the case for specimens from the Nova Scotia continental slope by Halliday (1970) and east of Grand Bank by Albikovskaya (1988). However, the time of spawning differed in Norwegian waters, ripe and spawning fish occurring most commonly in May and June (Gjøsæter, 1981).
Fecundity averaged 583 eggs (N = 97, range = 140–1098) in fish of 26–70 mm in length. These estimates are lower than those of Gjøsæter (1981), who found that specimens collected off Norway had an average fecundity of 781 eggs (N = 28, range = 162–1940) in fish of 45–70 mm. Fecundity was significantly correlated with fish length in both cases.
Sameoto (1988) found that the diet of B. glaciale over the Nova Scotia continental slope, adjacent to the present sampling area, was even more dependent on copepods than was the case in present data. The dominant food items in his collections were Calanus species. Diet studies of B. glaciale off eastern Grand Bank-Flemish Cap (Albikovskaya, 1988; García-Seoane et al, 2013) and in the western Labrador Sea (Pepin, 2013) also found Calanus, particularly C. finmarchicus, to be predominant, but sampling in these studies was also conducted along the continental slope. The most likely explanation for the absence of Calanus in the diet of the specimens examined in the present study is that members of this genus did not occur in the oceanic waters sampled. The minimum in stomach fullness in winter samples may indicate reduced feeding opportunities during that season.
Ceratoscopelus maderensis
Ceratoscopelus maderensis has a North Atlantic temperate distribution. Although initially thought to inhabit semi-subtropical waters also (Backus et al., 1977), subsequent evidence of the importance of cold-core rings in transporting Slope Sea species south into the Sargasso Sea caused Backus and Craddock (1982) to conclude that the species was better classed as temperate only, a conclusion supported by Karnella (1987), who caught only juveniles in sampling off Bermuda.
Themelis and Halliday (2012) found that this species ranked first in abundance in catches made in the temperate waters south-west of the LSW/WSW boundary, but found also that it ranked second in catches from the colder water to its northeast. The present analysis of data from standard night tows shows that there was a differential in density between water masses of about five to one, on average, in WSW vs. LSW. The differential was less in summer (June–October) than in winter. Few specimens of this species were caught in deep day tows, so standard night tows can be taken as reflective of the population as a whole. Occurrences in LSW were not limited to the area immediately adjacent to the boundary with WSW but were wide-ranging within the LSW area, as shown by the initial exploratory surveys, and previously by McKelvie (1985, a & b). Occurrences were common in McKelvie’s (1985b) samples from the Gulf Stream also, but few were caught in his samples from the Newfoundland Basin, east of Grand Bank.
There was a dichotomy in the size compositions between animals caught in WSW vs. LSW, those in WSW being generally less than 50mm in length and those in LSW being larger than that. The largest specimen recorded, at 75 mm, although caught in WSW, was taken close to the WSW/LSW boundary. Large C. maderensis have been reported previously as occurring also along the continental slope. Halliday and Scott (1969) caught fish as large as 70 mm along the Scotian Shelf slope, adjacent to the present sampling area. More significant, however, are the reports of Backus et al. (1968) of shoals of 52–73 mm fish over the continental slope south of New England (39°48'N, 70°33'W), and of Gartner et al. (2008) observing near-bottom aggregations of 54–74 mm fish over the continental slope off Cape Hatteras. Thus, large specimens appear to be associated with marginal environments.
In a study of Northeast Atlantic populations of this species, Linkowski et al. (1993) determined a life-span of two years, with a high mortality occurring after spawning in the first year. They noted also some geographic segregation, younger fish being largely absent from the northern and northeastern parts of their sampling area. The largest fish they recorded was 76 mm. Present data indicate that the life history in the northwestern Atlantic population exhibits a pattern very similar to that in the northeastern Atlantic, showing a segregation of age groups, those caught in WSW being 1 yr. olds and in LSW being primarily 2 yr. olds.
The reproductive contribution of the LSW component of the population is not clear. The large females caught in LSW matured sexually and contributed to spawning. However, those fish examined were from samples taken immediately adjacent to the LSW/WSW boundary and were not necessarily representative of the LSW population as a whole. Post-larval specimens occurred in LSW samples irregularly and only in small numbers, and these could have been transported from WSW due to mixing in the boundary area. Historical ichthyoplankton surveys showed larval occurrences over the continental slope from 64°W (the western end of the Scotian Shelf) south to Cape Hatteras. Only a few specimens were taken, late in the spawning season (Sept.–Oct.), from the more northeastern part of the Scotian Shelf adjacent to the present sampling area, suggesting that there was little spawning activity in that area. It is possible that those large animals caught in the central and eastern parts of LSW, and along the Nova Scotia continental slope, are expatriates, making no reproductive contribution, as described by Zurbrigg and Scott (1972) for Northwest Atlantic populations of Myctophum punctatum.
There are no studies of the diet of C. maderensis in the NW Atlantic comparable to present data (although Podrazhanskaya (1993) gave some general information from a sample taken east of Grand Bank that is not in conflict with present results). There was a fairly strong commonality in diet with B. glaciale, the similarity being about 65%. However, this is an overestimate as the comparison includes data at taxonomic levels above species. There was some seasonal variation in stomach fullness but it was less marked than that observed in B. glaciale.
Acknowledgements
We are grateful for the collaboration of many scientists and students in sample collection and particularly those from the Atlantic Reference Centre, St. Andrews, NB who provided also storage and work facilities. We are particularly grateful to Carla Dale (DFO) who was responsible for the logistics of at-sea sampling, served as chief scientist on several cruises, and developed systems for electronic data storage and retrieval. We thank also Wallace Morse, National Marine Fisheries Service, Northeast Fisheries Center, for providing access to electronic records of USA ichthyoplankton survey data for the study species, and also our colleagues P. Hurley and M. Showell for their comments on an earlier draft. The comments of two independent reviewers are also much appreciated.
References
ALBIKOVSKAYA, L. K. 1988. Some aspects of the biology and distribution of glacier lanternfish (Benthosema glaciale) over the slopes of Flemish Cap and eastern Grand Bank. NAFO Sci. Coun. Studies, 12: 37‒42.
BACKUS, R. H. and J. E. CRADDOCK. 1977. Pelagic faunal provinces and sound-scattering levels in the Atlantic Ocean. In: Ocean Sound Scattering Prediction. Andersen, N.R. & Zahuranec, B.J., Plenum Press, New York & London, p. 529‒547.
1982. Mesopelagic fishes in Gulf Stream cold-core rings. J. Mar. Res., 40, Supplement: 1‒20.
BACKUS, R. H., J. E. CRADDOCK, R. L. HAEDRICH, and B. H. ROBISON. 1977. Atlantic mesopelagic zoogeography. In: Fishes of the western North Atlantic, Sears Foundation for Marine Research, Memoir 1: 266‒287.
BACKUS, R. H., J. E. CRADDOCK, R. L. HAEDRICH, D. L. SHORES, J. M., TEAL, A. S. WING, G. W. MEAD, and W. D. CLARKE. 1968. Ceratoscopelus maderensis: peculiar sound-scattering layer identified with this myctophid fish. Science, 160: 991‒993. doi.org/10.1126/science.160.3831.991
CSANADY, G. T. and P. HAMILTON. 1988. Circulation of slopewater. Continental Shelf Research, 8: 565‒624. doi.org/10.1016/0278-4343(88)90068-4
GARCÍA-SEOANE, E., P. DALPADADO, and A. VÁZQUEZ. 2013. Feeding ecology of the glacier lanternfish Benthosema glaciale (Actinopterygii, Myctophidae) in the Flemish Cap (North Atlantic Ocean). Hydrobiologia, 717: 133‒146. doi.org/10.1007/s10750-013-1579-5
GARTNER, J. V., K. J. SULAK, S. W. ROSS, and A. M. NECAISE. 2008. Persistent near-bottom aggregations of mesopelagic animals along the North Carolina and Virginia continental slopes. Mar. Biol., 153: 825‒841. doi.org/10.1007/s00227-007-0855-1
GJØSÆTER, J. 1973. The food of the myctophid fish, Benthosema glaciale (Reinhart), from western Norway. Sarsia, 52: 53‒58.
1981. Growth, production and reproduction of the myctophid fish Benthosema glaciale from western Norway and adjacent seas. FiskDir. Skr. Ser. HavUnders., 17: 79‒108.
HALLIDAY, R. G. 1970. Growth and vertical distribution of the glacier lanternfish, Benthosema glaciale, in the northwestern Atlantic. J. Fish. Res. Bd. Can., 27: 105‒116. doi.org/10.1139/f70-011
HALLIDAY, R. G. and W. B. SCOTT. 1969. Records of mesopelagic and other fishes from the Canadian Atlantic with notes on their distribution. J. Fish. Res. Bd. Can., 26: 2691‒2702. doi.org/10.1139/f69-261
HALLIDAY, R. G., D. E. THEMELIS, C. E. DALE, and G. D. HARRISON. 1995. Oceanographic conditions off the Scotian Shelf during mesopelagic resource inventory cruises, 1984‒89. Can. Manuscr. Rep. Fish. Aquat. Sci., 2327: 303 p.
HISLOP, J. R. G. 1970. Preliminary investigations on the pelagic O-group phase of some demersal gadoids. ICES C.M. 1970/F: 12, 5p.
KARNELLA, C. 1987. Family Myctophidae, lanternfishes. In: Biology of midwater fishes of the Bermuda Ocean Acre. Gibbs Jr., R. H. and Krueger, W. H. (Eds.) Smithsonian Contributions to Zoology, 452: 51‒168.
KAWAGUCHI, K. and J. MAUCHLINE. 1982. Biology of myctophid fishes (Family Myctophidae) in the Rockall Trough, Northeastern Atlantic Ocean. Biol. Oceanogr., 1(4): 337‒373.
LINKOWSKI, T. B., R. L. RADTKE, and P. H. LENZ. 1993. Otolith microstructure, age and growth of two species of Ceratoscopelus (Osteichthyes - Myctophidae) from the eastern North Atlantic. J. Exp. Mar. Biol. Ecol., 167: 237‒260. doi.org/10.1016/0022-0981(93)90033-K
MCKELVIE, D. S. 1985a. Discreteness of pelagic faunal regions. Marine Biology, 88: 125‒133. doi.org/10.1007/BF00397159
1985b. The mesopelagic fish fauna of the Newfoundland Basin. Can. J. Zool., 63: 2176‒2182. doi.org/10.1139/z85-321
MORSE, W. W., M. P. FAHAY, and W. W. SMITH. 1987. MARMAP surveys of the continental shelf from Cape Hatteras, North Carolina, to Cape Sable, Nova Scotia (1977‒84). Atlas No. 2. Annual distribution patterns of fish larvae. NOAA Technical Memorandum NMFS-F/NEC-47.
OʼBOYLE, R. N., M. SINCLAIR, R. J. CONOVER, K. H. MANN, and A. C. KOHLER. 1984. Temporal and spatial distribution of ichthyoplankton communities of the Scotian Shelf in relation to biological, hydrological, and physiographic features. Rapp. P.-v. Reun. Cons. int. Explor. Mer, 183: 27‒40.
OLIVAR, M. P., A. BERNAL, B. MOLÍ, M. PEÑA, R. BALBÍN, A. CASTELLÓN, J. MIQUEL, and E. MASSUTI. 2012. Vertical distribution, diversity and assemblages of mesopelagic fishes in the western Mediterranean. Deep-Sea Res. Part I Oceanogr. Res. Pap. 62: 53–69. doi.org/10.1016/j.dsr.2011.12.014
PEPIN, P. 2013. Distribution and feeding of Benthosema glaciale in the western Labrador Sea: Fish-zooplankton interaction and the consequence to Calanoid copepoid populations. Deep-Sea Research, 175: 119–134. doi.org/10.1016/j.dsr.2013.01.012
PODRAZHANSKAYA, S. G. 1993. Feeding habits of mesopelagic species of fish and estimation of plankton graze in the Northwest Atlantic. NAFO Sci. Coun. Studies, 19: 79–85.
SAMEOTO, D. 1989. Feeding ecology of the lantern fish Benthosema glaciale in a subarctic region. Polar Biol., 9: 169–178. doi.org/10.1007/BF00297172
1988. Feeding of lantern fish Benthosema glaciale off the Nova Scotia Shelf. Mar Ecol. Prog. Ser., 44: 113–129. doi.org/10.3354/meps044113
THEMELIS, D. E. 1996 MS. Variations in the abundance and distribution of mesopelagic fishes in the Slope Sea off Atlantic Canada. PhD Thesis, Dalhousie University, Nova Scotia, Canada, xii+203p.
THEMELIS, D. E. and R. G. HALLIDAY. 2012. Species composition and relative abundance of the mesopelagic fish fauna in the Slope Sea off Nova Scotia, Canada. Northeastern Naturalist, 19(2): 177–200. doi.org/10.1656/045.019.0204
WORTHINGTON, L. V. 1964. Anomalous conditions in the Slope Water area in1959. J. Fish. Res. Bd. Can. 21: 327–333.
ZURBRIGG, R. E. and W. B. SCOTT. 1972. Evidence for expatriate populations of the lanternfish Myctophum punctatum in the northwest Atlantic. J. Fish. Res. Bd. Can., 29: 1679–1683. doi.org/10.1139/f72-267
|