Introduction
Haddock (Melanogrammus aeglefinus) is one of the most abundant species in the ichthyofauna of the Rockall Bank. Despite being of great commercial importance, the biology of this species, including aspects of its reproduction, has not thoroughly been investigated, which greatly complicates the development of effective measures for the regulation of its fishery. The earliest recorded research on the reproductive biology of Rockall haddock dates back to 1969–1970. Based on the analysis of summer data, Shestov and Blagodelskay (1971) concluded that haddock in this area reach sexual maturity at a length of not less than 40 cm. Shortly after their findings, Blacker (1971) established, based on his own observations, that haddock were maturing at a length of not less than 30 cm and as 4 year-olds. The results of surveys conducted by Soviet research vessels in 1972–1973 showed that spawning of haddock on the Rockall Bank occurs in April–May between 57° N latitude and at depths below 200 m (Chuksin and Gerber, 1976). Shestov (1977) reported that pre-spawning and spawning haddock were widely distributed on the Rockall Bank in March–April within the depth range of 150–350 m, with the highest concentrations being found in the central part of the bank at depths of 150–170 m. He also showed that haddock matured as 5 year-olds and that the fecundity in 4 year-olds at a length of 29–31 cm was, on average, 214 thousand eggs. According to Shestov (1977), the egg diameter in pre-spawning and spawning females varied from 0.4 to 1.8 mm.
Some useful complementary information to published studies was provided in March–April 1977 by the research vessel "Odissey" and the exploratory vessel "Parallax." According to the survey results, the bulk of catches (99–100%) on the southwest slope of the bank were made up by mature haddock. Thus, practically all individuals larger than 23 cm were at the pre-spawning or spawning stage.
In 1978, the introduction of the 200-mile zone around Rockall by the UK temporarily halted Russian research activities in the area. Studies on Rockall haddock biology continued only in the west-European countries. These studies intensified after 1985 when Scotland initiated regular demersal trawl surveys on the Rockall Bank aimed at assessing the status of haddock in the area.
Russian investigations on the Rockall Bank resumed their studies in the late 1990s, when the southwestern part of the bank was again designated as international waters (Vinnichenko and Khlivnoy, 2006). In recent years, the intensity of research has increased along with the development of regulatory measures for fisheries under the NEAFC framework. A significant part of the research work has been devoted to studying the reproductive cycle, maturation rate, fecundity and spawning features (Khlivnoy, 2005; Vinnichenko and Sentiabov, 2005; Khlivnoy, 2006; Vinnichenko and Khlivnoy, 2006; Vinnichenko and Khlivnoy, 2007; Arhipov and Mamedov, 2007). Some of this research has also been conducted by scientists from the Marine Laboratory in Aberdeen, Scotland (ICES, 2003).
In addition, studies of the reproductive biology are available for haddock of other areas (Hawkins, 1967; Sonina, 1973; Sonina, 1976; Hislop et al., 1978; Alekseeva and Tormosova, 1979; Hislop and Shanks, 1981; Hislop and Bell, 1987; Hislop, 1988; Blanchard et al., 2003; Filina, 2004; Rideout et al., 2005).
According to most researchers, the group of haddock from the Rockall Bank is an isolated population (Chuksin and Gerber, 1976; Shestov, 1977; Blacker, 1982; Vinnichenko and Khlivnoy, 2007). Haddock are of a smaller size, compared to those in other areas and differ from other populations in terms of rates of growth, feeding and population dynamics (Shestov, 1977; Blacker, 1982; Khlivnoy, 2006; Vinnichenko and Khlivnoy, 2007). The main objective of the present study is to summarise and analyse the results of previous studies on the biology of the Rockall haddock as well as to provide new information in relation to the reproductive biology of this stock. In addition, this study aims to compare the reproductive biology of this population to that of other populations of haddock.
Material and Methods
The present study utilized data collected during 42 surveys conducted by Russian exploratory, research and commercial vessels on the Rockall Bank in the period 1958–2006. The investigations covered the depths from 140 to 580 m. The bottom trawl was towed at 3.0–3.5 knots for 0.5–6.0 hr at station. Collection and processing of primary biological material were conducted according to working instructions and techniques of PINRO (Anon, 2001). During the entire period of Russian research on haddock there were: 223 754 body length measurements, 23 395 maturity determinations, and 20 932 field feeding analyses. These data were combined with data from Russian, ICES and Scottish research publications.
Haddock maturity was determined according to the following scale:
II - immature;
III - maturing;
IV - pre-spawning;
IVa (IV-V) - pre-spawning (in females: hydrated hyaline oocytes present but having not spawned batches of sexual products);
V - spawning;
Va (VI-IV) - having spawned one or several batches of sexual products, but not having completed spawning (in females: no hydrated oocytes present);
Vb (VI-IV-V) - having spawned one or several batches of sexual products (in females: hydrated hyaline oocytes present);
VI - post-spawning;
VI – II - post-spawning recovery.
Stages Va and Vb are additional detailed stages of spawning.
The proportion of mature fish (PML) in each size group was determined and modeled using a logistic curve described by the following equation:

where L is the size group, ML50 is the fish length, under which 50% of this size fish were mature, and b is a constant, reflecting the angle of curve slope.
Samples of haddock ovaries and testes for microscopic examination were collected on the Rockall Bank in 2004–2006 (Table 1, Fig. 1). Whole gonads were fixed in Bouin’s fluid or in 10% formalin. A small piece from the middle part of a gonad was taken for histological examination. Ovarian and testicular samples were processed using standard histological methods by dehydration through alcohol, and clearing in xylene and xylene-paraffin (Roskin and Levinson, 1957). Prepared paraffin sections, 5–7 µm thick, were stained with iron hematoxylin. They were examined under different magnifications using an Olympus BX 41TF microscope. In total, 234 gonads of haddock were subject to histological examination. The gonadosomatic index (GSI) was calculated as the ratio of gonad weight to total fish weight and expressed in percent. For studying the seasonal cycle and maturation rates, data obtained from field observations were also used.
To study fecundity, ovaries of fish 24–71 cm in length (130–1 480 g in weight and 2–9 years old), were collected on the Rockall Bank in April 2004 and March 2005 (Fig. 1). In total, 58 female haddock gonads were examined. The examination was carried out according to the methods of Spanovskaya and Grigorash (1976): individual potential fecundity (IPF) of haddock was determined by the standard weight method in which the numbers of eggs in the pre-spawning stage IV ovary samples of 50–100 mg weight were counted and raised to the whole ovary weight. Eggs diameter was also measured under a microscope. The criteria to identify eggs for fecundity estimation (separation of previtellogenic from developing oocytes) was based on size (>200–300 µm) and was confirmed by histological examination. To estimate batch fecundity the number of hydrated hyaline oocytes in follicles were counted (stage IVa). The number of batches was determined as the ratio of IPF to batch fecundity. Absolute potential fecundity (APF) was calculated as a mean value of individual potential fecundities per age.
Fish age was determined from otolith rings in the central section using a binocular microscope at ×16 with reflected light after clearing in alcohol-glycerin solution. Opaque summer and hyaline winter growth zones together were considered as an annual increment.
Results
Oocyte maturation
In Rockall haddock, as well as in other gadoids, development of vitellogenic follicles from previtellogenic follicles to form the potential fecundity begins with the occurrence of vacuoles on the periphery of cytoplasm (Fig. 2a). The oocyte size at the start of the period of major growth is 190–240 µm (early stage III). The nucleus is located in the centre, light in colour, and the nuclear envelope is scalloped. Numerous nucleoli are located near to the nuclear envelope. Fine granules of yolk and the vacuoles in the peripheral zone of the cytoplasm appear almost simultaneously. Increasing in size and quantity, the yolk moves to the nucleus. At the same time, the size of oocytes increases and reaches 300–350 µm. The oocytes enter a phase of intensive trophoplasmatic growth (late stage III) (Fig. 2b). At this stage, maximum accumulation of nutrients takes place. In the yolk-filled phase (stage IV), the oocyte reaches 550–700 µm and the size of yolk granules is 6–10 microns (Fig. 2c). The nucleus begins to be displaced from the center. The next stage in the development of oocytes is homogenisation and hydration of the yolk. The hydrated oocytes (stage V) reach a size of 1 000–1 600 µm and are ready for ovulation (Fig. 2d).
Sexual cycle and spawning pattern in females
Post-spawning females start to appear in March–April and form large concentrations in May–June. After spawning (stage VI), the ovary contains many large empty follicles and oocytes at an early phase of previtellogenic development (Fig. 3a, b). The post-spawning recovery appeared to be a quick process because fish in this stage soon disappeared from the catches. The size of empty follicles is significantly reduced due to resorption and the number of oocytes with protoplasmatic growth increases. The ovary then passes to stage VI – II. The residuals of post-ovulatory follicles serve as a reliable criterion to identify fish that had participated in the last spawning season. One more indication of completed spawning is the remnants of isolated large mature vitellogenic follicles that appear to persist till September and the thickened ovary wall (Fig. 3b,c).
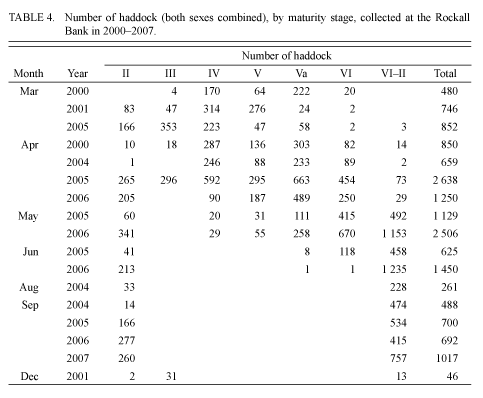
In the samples taken in early- and mid-September 2004 and 2005, no ovarian maturation (stage III) was observed. According to the results from visual observations in January 1980 and December 2001, more than half of the adult individuals had maturing gonads (stage III). As the research results show, stage III can occur until March and in some individuals until April (Tables 2, 3 and 4).
Recruitment of vitellogenic follicles from the precursory population of previtellogenic follicles appears to occur synchronously with a gap appearing between the two populations suggesting that fecundity was determinate. However, later in maturation the vitellogenic follicles show more asynchronous development because the size range between the smallest and largest part of the population becomes more pronounced. Batch production from the vitellogenic group follows and the size of the vitellogenic population reduces as spawning progresses (Figs. 3d,e and 4b,c).
According to field observations in 2002–2006 mass spawning of haddock was recorded in the second half of March–first half of May. In 2005, the first specimens with a batch of hydrated follicles (stage IVa) appeared in the first half of March (Figs. 3e and 4c). Batch fecundity comprised 5.3–9.5% of the potential fecundity indicating that between 11 and 19 spawning events were required to exhaust the supply of vitellogenic follicles until the female was spent. Hydrated hyaline oocytes that are ready to be spawned are largest in size (Fig. 4d). After the ovulation of the first and following batches an ovary goes into stage Va (Figs. 3f and 4e) and then, in process of hydration of sexual cells, in stage Vb (Fig. 4f,g).
The remnants of postovulatory follicles on histological preparations indicate that some quantity of eggs have already ovulated in batches spawned previously. During the course of spawning there is a reduction in the proportion of oocytes in the initial phase and increase in number oocytes in the final phase of trophoplasmatic growth (Fig. 4e,f,g).
The number of batches is similar, and batch fecundity increases with female size in proportion to the increase in absolute potential fecundity. The spawning is completed by ovulation of all hydrated oocytes (stage VI).
One of the important parameters describing an ovary condition is gonadosomatic index (GSI). The maximal values of GSI were 12.0–19.6% and have been observed for pre-spawning individuals at stage IVa in March–April. During the process of spawning, GSI is gradually reduced and spent females (stage VI) in May have a GSI corresponding to 0.9–2.4%. Minimal values of GSI of mature females (0.4–0.8%) have been observed at the end August–September when basically post-spawning regeneration has come to an end and maturing has not yet begun.
Dynamics of gonad development in male haddock
According to the field observations collected in December–January, all the mature males had ripening testes (stage III). Histological examination of gonads showed that in early March, the testes in some individuals were characterized as pre-spawning (Fig. 5a). In the testes, sperm cysts already contained mature spermatozoids, however, spermatid and spermatocyte cells that represent an earlier stage of development were also found. Samples collected in April had both spawning and post-spawning males (Fig. 5b). In the May sample, all the mature males were post-spawning (Fig. 5c), as characterized by the presence of residual spermatozoa. These resorbing sexual product were found in testes till September (Fig. 5d). The presence of these can be used to enable the separation of mature males participating in spawning from the immature individuals.
Maturity
Visual observations in 2000–2002 and 2004–2006, showed that practically all haddock of length 25 cm and larger were mature. At a length of 24 cm more than half of individuals were mature (Fig. 6). In March–April 1977 practically all individuals with length larger than 23 cm (Tables 2 and 3) were mature. In the spring of 2000, the smallest mature haddock were 21 cm in length. In March–April the length at which more than half of individuals were mature was 21.1 cm (Fig. 6).
In catches from March 2001, mature individuals with length 18–20 cm (Fig. 6) were observed. At the same time, some individuals of 26–27 cm in length remained immature, and the length at which more than half of individuals became mature, has increased to 22.4 cm. In April 2002, at this length only 14% of fish were mature, however all individuals in length of 25 cm and larger were mature. In March–April 2004, mature fishes were again recorded at lengths of 20–21 cm and in March–April 2005 at lengths of 18–19 cm (Fig. 6). In March 2005, GSI of males rose from 1.9 to 3.3%, females rose from 4.1 to 11.1%, and has on the average been 2.1% for males and 7.0% for females. Thus more than half the individuals at a length of 23.8 cm were mature (Fig. 6).
For all data collected from 2000–2006, half of the males were mature at a length of 22.6 cm and the length at which half of females were mature was 23.5 cm (Fig. 7).
The data from a histological examination of haddock gonad samples collected in 2004–2006 showed that almost all females (92.3%) of length 25 cm, and all males (100.0%) of length 24 cm and larger were mature. More than half of females were mature at 24 cm (Fig. 8). The minimum length of mature females in the sample was 22 cm and 18 cm for males. It is concluded, that sexual maturation normally occurs at 2 years of age (ICES, 2003).
Visual examination of haddock gonads collected in the spawning period 2000–2006 showed that ML50 = 23.1 cm, according to histological examination ML50 = 23.0 cm (Fig. 8).
Of particular interest are results of a comparison of histological and visual estimation of maturity status of haddock gonads collected in the post-spawning period. From a visual examination of 38 gonads, collected in August–September 2004, there were 16 immature and 22 post-spawning fish. Histological examination showed that there were only four immature fish in the sample and the other 34 females took part in spawning in spring (Fig. 9). The length of 12 fish which were erroneously classified as immature ranged 25–35 cm (27.7 cm on average).
Areas and periods of spawning
At the end of March 1977, the majority of individuals were in pre-spawning condition (Table 2). Active spawning was recorded in the first half of April. During this period, 57% of individuals were in a condition of spawning, and 25% had finished spawning (Table 3).
In 2000, spawning of haddock was found during the period from March until May. On a site located outside the 200-mile zone, spawning haddock occurred in a range of depths 185–290 m. The most intensive spawning was recorded in the first half of April. At the end of the month, the proportion of post-spawning individuals in catches had increased to 52%. During the spawning season some pre-spawning fishes were observed on the spawning area. At the beginning of June all non-immature individuals were in a post-spawning condition.
In 2001, on the southwest slope of the bank, spawning began in the middle of March at depths from 180 to 350 m, with the most dense concentrations of haddock recorded in depths of 200–300 m. The proportion of spawning females and males around 20 March was 27% and 19%, respectively. By the end of March their quantity had increased to 64 and 66%, respectively. In April–May research here was not pursued. In June–July, the density of concentrations had decreased considerably. The main part of the haddock population had finished spawning and had left the area.
In the same area in the middle of April 2002, 45% of the catches consisted of spawning individuals. Some 16% of the fish had spawned one or several batches of eggs and were preparing to spawn another batch, and 14% of individuals were in post-spawning recovery. Additionally, some post-spawning females were observed.
In 2004, spawning of haddock in the southwest of the bank began about 10–20 March. By the end of March, more than 90% of individuals were in spawning or pre-spawning condition. In the first half of April, about 27% of males had finished spawning (stage VI).At the same time about 77% of females had only started spawning and 3% had finished spawning. By 20 May, spawning had practically ended.
In March 2005, spawning haddock were caught in practically all areas of the bank, however the most active spawning was on the southwest part of the bank at depths of 180–300 m. During this period, the majority of the haddock stock was concentrated here. Spawning of haddock began about the middle of March. By the end of the month, more than 90% of individuals were in spawning or pre-spawning condition and part of the population had finished spawning. In April, 20% of individuals had finished spawning. In May, the majority of individuals had finished spawning, in June only some spawning individuals were recorded.
In 2006, 54% of individuals were spawning in the second half of April. By the beginning of May, 52% of males and 62% of females had finished spawning. Spawning was basically completed by the second half of May.
Sexual structure
In March–April 2000, catches were dominated by females on the southwest slope banks (Table 5). In 2001, the quantity of females in the March catches was greater than males (1.0:1.3), at the end of June–July the relative proportion of females had increased (1.0:1.6), and females still dominated the catches during August (Table 5). In 2003, females in May–June were slightly more numerically prevalent (1.0:1.1), whereas in July–August males dominated (Table 5). In 2004, the catches in March–April contained more females, whereas in May and August–September there were more males. In 2005, females prevailed only in March and September. In 2006, the ratio of sexes was approximately equal during all periods of research (Table 5).
Fecundity
In accordance with the data from work carried out in 2004–2006 the individual potential fecundity of females with a length of 24–71 cm and with a weight of 130–3 480 g of 2–9 year-olds varied from 44 thousand to 2.3 million eggs. Females with a length of 26 cm, with the weight of 130 g at the age of 2 years had the minimum fecundity, the one with length 71 cm, weight of 3 480 g and 9 years old had the maximum fecundity. Absolute potential fecundity (APF) increased with age: it was 78 thousand eggs in first spawning fish of 2 years old, 170 thousand eggs in 3 year olds, 340 thousand eggs in 4 year olds, 555 thousand eggs in 5 year olds, 716 thousand eggs in 7 year olds, and 947 eggs in 8 year olds (Fig. 10a). These results correspond well with results of the previous research (Shestov, 1977).
The dependencies of absolute potential fecundity APF on age, length and weight (Fig. 10) are described by the equations:



where APF is absolute potential fecundity, a is age in years, L is length in cm, W is weight in g. The closest correlation (r2 = 0.92) is between fecundity and weight of a haddock (Fig. 10c).
Average batch fecundity in thousands of eggs was 4.3 for 3 year-olds, 17.0 in 4 year olds, 36.5 in 5 year olds, 40.1 in 8 year olds. In some individuals the batch varied from 5.3 to 9.5% of the total potential fecundity. This did not vary between years, and was 6.1% for 3 year-olds, 5.3% for 4 year olds, 7.4% for 5 year olds, and 6.2% for 8 year olds. Haddock produces 11–19 batches over the course of a spawning season. The number of batches does not depend on the age of fish. The vitellogenic oocytes had the mean diameter of 663 µm (S.D. = 112 µm) and hydrated oocytes 1 417 µm (S.D. = 102 µm).
Discussion
According to most researchers, the group of haddock from the Rockall Bank is an isolated population (Chuksin and Gerber, 1976; Shestov, 1977; Blacker, 1982; Vinnichenko and Khlivnoy, 2007). Rockall haddock are of a smaller size when compared to haddock from other areas. The bulk of the population is made up of individuals 20–35 cm long, the portion of larger haddock is minor (Khlivnoy, 2005, 2006; ICES, 2005a; ICES, 2007; Vinnichenko and Khlivnoy, 2007). It has previously been established, that the haddock of this area differ from other populations in terms of rates of growth, feeding and population dynamics (Shestov, 1977; Blacker, 1982; Khlivnoy, 2006; Vinnichenko and Khlivnoy, 2007). Results of the present research suggests, that the haddock of this population also have a number of particular features with respect to rates of sexual maturity, character of spawning and fecundity.
As microscopic examination showed, in the period of trophoplasmatic growth, the oocytes of Rockall haddock had the same development phases as those of the other Gadidae (Sorokin, 1957; Alekseeva and Tormosova, 1979). These are the phases of vacuolization and primary accumulation of yolk, intensive trophoplasmatic growth, yolk-filled oocyte and of hydration (Fig. 2). All the oocytes of haddock from both the Rockall area and the North Sea (Alekseeva and Tormosova, 1979) for spawning in the nearest spawning period enter trophoplasmatic growth in autumn. The Rockall haddock oocyte development is asynchronous process.
Similar spawning processes occur in haddock from the North Sea where the quantity of eggs in the first ripened batch makes 7–10% of the number of oocytes in trophoplasmatic growth, and all females spawn 10–14 batches of eggs (Alekseeva and Tormosova, 1979).
The Rockall haddock population has an early rate of maturing. The results from Russian research on haddock indicated that mass sexual maturation occurs at the 2 years of age with a length of 25 cm. These data agree well with the results of recent Scottish research which indicated that the majority of fish become mature as 2 year-olds (ICES, 2003).
The bottom water temperature across the areas of distribution of haddock varies over a wide range, from 3°C in Barents Sea and up to 11°C in Celtic Sea (Table 6). The populations with early age of maturity live in warmer waters, and populations living in areas with colder bottom water temperatures mature at an older age (Table 6). The latest maturing population is that of the Northeast Arctic haddock living in waters with bottom temperature 3–4°С. The fish of this population become mature at approximately six-years-old. Haddock become mature at the age of 3–4 years in the rather warm water areas around Iceland, Faroe Islands and the North Sea. The haddock living in areas to the West from British Isles where the temperature of the water in the benthic layer is 10–11°С have the earliest age at maturation and on average maturation occurs at 2 years of age (Petrie and Drinkwater, 1993; Malmberg, 1999; Drinkwater, 2005; ICES, 2005b, c, d, e, f). The Rockall haddock lives in similar temperature conditions and, probably, for this reason it also matures at early ages (ICES, 2003).
A comparative analysis of the results from visual and histological estimation of haddock maturity indicated that the ovary maturity stages were determined in sea conditions very accurately in pre-spawning and spawning periods (March–May) when the gonads of mature and immature fish significantly differed in appearance. However, in the post-spawning period, the results from field visual determination of haddock female gonad stage was prone to error compared to the histological staging. Evidently, in the field, in order to obtain more reliable data, female maturity should be determined in pre-spawning and spawning periods. The unreliability of field visual maturities determined during the post-spawning period could potentially be used to explain changes in fish size at maturity in the 1960s and 1970s. For example, difficulties associated with determining maturity of haddock during the summer (post-spawning) season of 1969–1970, likely led to an over-estimate of length at sexual maturity. At the same time, practically all individuals with length more than 23 cm were determined as mature in March–April, 1977 (the spawning period) (Tables 2 and 3) and this corresponds well with the modern data.
There was some inter-annual variation in spawning period but mainly spawning takes place in March–May and mainly on the southwest slope of Rockall bank. During this period the females, as a rule, numerically prevail over males in this area (Table 5). Towards the end of spawning the relative quantity of females usually decreases. During the post-spawning period (June–September) in the southwest banks males are slightly more abundant or both sexes occur in approximately equal numbers.
The absolute potential fecundity of Rockall haddock is close to those in the North Sea population but is lower than the fecundity of the Northeast Arctic population. Alekseeva and Tormosova (1979) give the fecundity of North Sea haddock as 118 thousand eggs for 3 year olds, 418 thousand eggs for 5 year olds and 660 thousand eggs for 7 year olds, while Barents and the Norwegian Seas it is 879 thousand eggs for 5 year-olds and 958 thousand eggs for 7 year olds (Sonina, 1973). Compared with these haddock stocks the fecundity at age is lower on the eastern Scotian Shelf. Blanchard, Frank and Simon (2003) reported that the fecundity of the eastern Scotian Shelf haddock was 123 thousand eggs for 5 year-olds and 146 thousand eggs for 6 year olds. The potential and actual fecundity may differ substantially depending upon the numbers of oocytes in the potential that are resorbed during the spawning season (Kjesbu et al., 1991; Armstrong et al. 2001; Hunter and Macewicz, 2003). Histological analysis would have to be used to select pre-spawning females and the criteria to identify eggs comprising the fecundity.Generally the mean diameter of the advanced stock of oocytes is the accurate measure of ovarian maturity (Hunter et al., 1989; Hunter et al., 1992; Gundersen et al., 2000; Kurita et al., 2003; Murua et al., 2003). The vitellogenic oocytes of Rockall haddock had the mean diameter of 663 µm and hydrated oocytes 1 417 µm.
The batch fecundity of Rockall haddock increased with age proportional to the increase in absolute potential fecundity but the number of batches does not depend on age of the fish. Results of the present research corresponds well with other studies of haddock. Alekseeva and Tormosova (1979) moreover reported that during spawning 10–14 batches of eggs were spawned. Hawkins et al. (1967) give 14 batches and Rideout et al. (2005) give 10–22 batches of eggs for haddock spawned in captivity.
Conclusions
The research, executed in recent years, improves the knowledge of reproductive biology of Rockall haddock and allows the following conclusions to be drawn:
- Asynchronous vitellogenesis and batch spawning are typical of the Rockall haddock.
- The minimum length of mature female in histological samples is 22 cm, and that of males is 18 cm.
- The majority of haddock mature at a length of 25 cm for females, and 24 cm for males. Average age at first maturity of both sexes was at 2 years of age.
- Rates of maturation of Rockall haddock are close to the populations of this species living on the continental shelf around the British Isles
- Data on haddock maturity in field conditions should be determined in pre-spawning and spawning periods. Conducting these studies during other seasons of the year increases the probability of mistakes.
- Haddock spawning mainly took place in March–May.
- Fecundity increases with age.
Results of the present research may be used in the development of regulatory measures for the Rockall haddock fishery.
References
ALEKSEEVA, E. I., and I. D. TORMOSOVA. 1979. Maturation, spawning and fecundity of haddock Melanogrammus aeglefinus (L.) from the North Sea. Vopr. ikhtiol., 19: 447–457. [in Russian]
ARMSTRONG, M. J. P., P. CONOLLY, R. D. M. NASH, M. G. PAWSON, E. ALESWORTH, P. J. COULAHAN, M. DICKEY–COLLAS, S. P. MILLIGAN, M. F. O'NEILL, P. R. WITTHAMES, and L. WOOLNER. 2001. An application of the annual egg production method to estimate the spawning biomass of cod (Gadus morhua L), plaice (Pleuronectes platessa L) and sole (Solea solea L.) in the Irish Sea. ICES J. Mar. Sci., 58: 183–203. doi:10.1006/jmsc.2000.1001
ANON. 2001. Instructions and methodical recommendations for gathering and processing of the biological information in areas of research. PINRO, Murmansk, 291 p.
ARKHIPOV, A. G., and A. A. MAMEDOV. 2007. Ichthyoplankton from the underwater Rockall–Hatton sea mounts. Vopr. ikhtiol., 47: 506–514. [in Russian]
BLACKER, R. W. 1972. Synopsis of biological data on haddock Melanogrammus aeglefinus (Linnaus) 1758. FAO Fisheries Synopsis, Rome, No 84, 50 p.
1982. Rockall and its Fishery. Laboratory Leaflet, No. 55, Lowestoft, 23 p. http://www.cefas.co.uk/publications/lableaflets/lableaflet55.pdf
BLANCHARD, J. L., FRANK, K. T., and SIMON, J. E. 2003. Effects of condition on fecundity and total egg production of eastern Scotian Shelf haddock (Melanogrammus aeglefinus). Can. J. Fish. Aquat. Sci., 60: 321–332. doi:10.1139/f03-024
CHUKSIN, YU. V., and E. M.GERBER. 1976. Soviet Fishery in the Rockall and Porqupine areas. Zaprybpromrazvedka. Kaliningrad, 8 p. [in Russian]
DRINKWATER, K. MS 2005. Cod stocks: winners and losers in the climate change sweepstakes. ICES 2005 newsletter, September 2005, Issue No. 42, p. 6–7. http://www.ices.dk/products/newsletters/ices42.pdf
FILINA, E. A. 2004. Histological examination on gonads cod and haddocks of the Barentseva sea in connection with the miss of spawning. In: Modern problems of physiology and biochemistry of water organisms. Petrozavodsk, Russia, p. 139.
GUNDERSEN, A. C., K. H. NEDREAAS, O. S. KJESBU, and O. T. ALBERT. 2000. Fecundity and recruitment variability of Northeast Arctic Greenland halibut during 1980–1998, with emphasis on 1996–1998. J. Sea Res., 44: 45–54. doi:10.1016/S1385-1101(00)00038-1
HAWKINS A. D., C. J. CHAPMAN, and D. J. SYMONDS. 1967. Spawning of haddock in captivity. Nature, 215: 915–925. doi:10.1038/215923a0
HISLOP, J. R. G., A. P. ROBB, and J. A. GAULD. 1978. Observations on effects of feeding level on growth and reproduction in haddock, Melanogrammus aeglefinus (L.) in captivity. J. Fish. Biol., 13: 85–98. doi:10.1111/j.1095-8649.1978.tb03416.x
HISLOP, J. R. G., and A. M. SHANKS. 1981. Recent investigations on the reproductive biology of the haddock, Melanogrammus aeglefinus, of the northern North Sea and the effects on fecundity of infection with the copepod parasite Lernaeocera branchialis. J. Cons. Int. Explor. Mer., 39: 244–251.
HISLOP, J. R. G., and M. A. BELL. 1987. Observations on the size, dry weight and energy content of the eggs of some demersal fish species from British marine waters. J. Fish. Biol., 31: 1–20. doi:10.1111/j.1095-8649.1987.tb05209.x
HISLOP, J. R. G. 1988. The influence of maternal length and age on the size and weight of the eggs and the relative fecundity of the haddock, Melanogrammus aeglefinus, in British waters. J. Fish. Biol., 32: 923–930.doi:10.1111/j.1095-8649.1988.tb05435.x
HUNTER, J. R., B. J. MACEWICZ, and C. A. KIMBRELL. 1989. Fecundity and other aspects of the reproduction of sablefish, Anoplopoma fimbria, in central California waters. Rep. CCOFI., 30: 61–72.
1992. Fecundity, spawning, and maturity of female Dover sole Microstomus pacificus, with an evaluation of assumptions and precision. Fish. B.-NOAA, 90: 101–128.
HUNTER, J. R., and B. J. MACEWICZ. 2003. Improving the accuracy and precision of reproductive information used in fisheries. Fisken og Havet, 12: 57–68.
ICES. 2003. Report of the Working Group on the Assessment of Northern Shelf Demersal Stocks, 2002. ICES CM 2003/ACFM:04, 634 p. http://www.ices.dk/products/CMdocs/2003/ACFM/ACFM0403.pdf
2005a. Report of the Arctic fisheries working group (AFWG), 19–28 April 2005. ICES CM 2005/ACFM:20, 578 p. http://www.ices.dk/products/CMdocs/2005/ACFM/ ACFM2005.pdf
2005b. Report of the Working Group on the Assessment of Northern Shelf Demersal Stocks, 2004. ICES CM 2005/ACFM:01, 722 p. http://www.ices.dk/products/CMdocs/2005/ACFM/ACFM0105.pdf
2005c. Report on the Assessment of Demersal Stocks in the North Sea and Skagerrak, 2004. ICES CM 2005/ACFM:07, 783 p. http://www.ices.dk/products/CMdocs/2005/ACFM/ACFM0705.pdf
2005d. Report of the North Western Working Group (NWWG), 2005. ICES CM 2005/ACFM:21, 615 p. http://www.ices.dk/products/CMdocs/2005/ACFM/ACFM2105.pdf
2005e. Report of the Working Group on the Assessment of Southern Shelf Demersal Stocks, 2004. ICES CM 2005/ACFM:03, 543 p. http://www.ices.dk/products/CMdocs/2005/ACFM/ACFM0305.pdf
2006. Report of the Working Group on the Assessment of Northern Shelf Demersal Stocks (WGNSDS), 2005. ICES CM 2006/ACFM:13, 803 p. http://www.ices.dk/products/CMdocs/2006/ACFM/ACFM1306.pdf
2007. Report of the Working Group on the Assessment of Northern Shelf Demersal Stocks (WGNSDS), 2007. ICES CM 2007/ACFM:22, 868 p. http://www.ices.dk/products/CMdocs/CM-2007/ACFM/ACFM2207.pdf
KHLIVNOY, V. N. 2005. Life history and seasonal migrations of main commercial Rockall fish species. In: Proceedings of the International Conference of RAS “Fish behaviour”. M. Borok (ed.). Aquaros, p. 530–536. [in Russian]
2006. New methodical approaches to recovery of catch structure and haddock stock assessment in the Rockall Bank area. Vopr. Rybolov., 7: 161–175. [in Russian]
KJESBU, O. S., J. KLUNGSOYR, H. KRYVI, P. R. WITTHAMES, and M. GREER WALKER. 1991. Fecundity, atresia, and egg size of captive Atlantic cod (Gadus morhua) in relation to proximate body composition. Can. J. Fish. Aquat. Sci., 48: 2333–2343. doi:10.1139/f91-274
KURITA, Y., O. S. KJESBU, and S. MEIER. 2003. Oocyte growth and fecundity regulation by atresia of Atlantic herring (Clupea harengus) in relation to body condition throughout the maturation cycle. J. Sea Res., 49: 203–219. doi:10.1016/S1385-1101(03)00004-2
MALMBERG, S. A., and J. Desert. 1999. Hydrographic conditions in the North Icelandic waters and annual air temperature in Iceland. ICES CM 1999/L:14, 21 p.
MURUA H., G. KRAUS, F. SABORIDO–REY, P. R. WITTHAMES, A. THORSEN, and S. JUNCUERA. 2003. Procedures to estimate fecundity of marine fish species in relation to their reproductive strategy. J. Northw. Atl. Fish. Sci., 33: 33–54. doi:10.2960/J.v33.a3
PETRIE, B., and K. DRINKWATER. 1993. Temperature and salinity variability on the Scotian Shelf and in the Gulf of Maine 1945–1990. J. Geophys. Res., 98: 20079–20089. doi:10.1029/93JC02191
RIDEOUT, R. M., E. A. TRIPPEL, and M. K. LITVAK. 2005. Effects of egg size, food supply and spawning time on early life history success of haddock (Melanogrammus aeglefinus). Mar. Ecol. Prog. Ser., 285: 169–180. doi:10.3354/meps285169
ROSKIN, G. I., and L. B. LEVINSON. 1957. Microscopic equipment. Moscow, 465 p. [in Russian]
SHESTOV V. P., Z. V. BLAGODELSKAY. 1971. Investigations of the haddock stock on the Rockall Bank in 1969 and 1970. ICES C.M. 1971/F:22, 5 p.
SHESTOV, V. P. 1977. Rockall haddock. Fishery biological resources of the North Atlantic and adjacent seas of the Arctic Ocean. Moscow, p. 344–346. [in Russian]
SONINA, M. A. 1973. Formation of year classes and exploitation of commercial fish stocks of the Barents and Norwegian Seas. Tr. PINRO, 33: 171–201. [in Russian]
1976. State of the Arcto–Norvegian haddock stocks and factors determining the population abundance. Tr. PINRO, 37: 129–150. [in Russian]
SOROKIN, V. P. 1957. Ovogenesis and sexual cycle of cod (Gadus morhua L.). Tr. PINRO, 10: 125–144. [in Russian]
SPANOVSKAYA, V. D., and V. A. GRIGORASH. 1976. On methods of estimating the fecundity of females of simultaneous and portional spawning. Standard methods of research on fish species within their areas. Part II. Mosklas Press, Vilnus, p. 54–62. [in Russian]
SYSOEVA, T. K., L. G. PTITSYNA, and L. V. GERASIMOVA. 976. Feeding, growth and survival of larvae and fry of the Barents Sea haddock. Tr. PINRO, 37: 129–150. [in Russian]
VINNICHENKO, V. I., and E. V. SENTYABOV. 2005. Distribution and migrations of haddock (Melanogrammus aeglefinus) in the Rockall Bank area. Vopr. Rybol., 6: 44–55. [in Russian]
VINNICHENKO V. I. and V. N. KHLIVNOY. 2006. Study of demersal fishes on Rockall Bank. Rybn. Khoz., 1: p. 21–39. [in Russian]
2007. Biology and fishery of haddock from the Rockall Bank. Vopr. Rybol., 8: 44–55. [in Russian]
|