Introduction
An understanding of the reproductive biology of a species is a central aspect of providing sound scientific advice for fisheries management. It is reproductive biology that in large part determines productivity and therefore a population’s resilience to exploitation or perturbation from other human activities. The importance of quantifying productivity in terms of reproductive potential (RP) and recruitment, as well as the difficulty in doing so, have long been recognized (Ricker, 1954; Hilborn and Walters, 1992).
Some aspects of fish reproductive biology such as reproductive strategy (e.g. batch vs total spawner, determinate vs indeterminate spawners) are a characteristic of the species and are fixed. However, many reproductive traits are highly plastic. Life history parameters such as maturity at size or age, sex ratio, fecundity and spawning time and duration, vary between populations of a species. Moreover, they can also vary temporally within a population, altering a population’s productivity or RP over time. Changes in these life history parameters involve trade offs in energy allocation between growth and reproduction (maturation and fecundity) and interactions with mortality (Rijnsdorp, 1990; Stearns, 1992). The optimization of fitness in fish must be accomplished through the solution of a complex multidimensional problem. These tradeoffs shape variation in reproductive biology and hence variation in RP.
To derive adequate estimates of RP, variation in reproductive biology needs to be taken into account. If RP is measured with error then this can obscure any stock recruit (S/R) relationship (Walters and Ludwig, 1981). By extension, if failure to incorporate variation in reproductive biology produces an index of RP that does not adequately reflect the population’s actual RP, the result can be an apparent lack of a S/R relationship. A number of studies have shown that failure to account for variation in reproductive biology can affect the perception of productivity and that spawning stock biomass (SSB) may not be the best measure of RP (Marshall et al., 1998; Morgan and Brattey, 2005; Scott et al., 2006).
Given the significance of S/R to scientific advice for fisheries management, it is important to produce the best possible estimates of RP. Marshall et al. (2003) provides examples of improvements in estimates of RP for a number of fish populations. This current paper provides an overview of variation in reproduction in commercial fish species. It examines the impact that taking such reproductive variability into account can have on scientific advice for fisheries management. Where possible, the overview is illustrated with examples analysing data available for American plaice (Hippoglossoides platessoides). Some suggestions for possible future research directions are also given.
Estimation of Reproductive potential
Age and size at maturity
Perhaps the best studied fish reproductive characteristic is maturity. There are many examples of variability in maturity at age and/or size. A variety of species has been shown to exhibit interpopulation differences in maturation. Some of these include bluegill sunfish (Lepomis macrochirus) (Belk, 1995); brook trout (Salvelinus fontinalis) (Hutchings, 1993); pumpkinseed sunfish (Lepomis gibbosus) (Fox, 1994); northern pike (Esox lucius) (Diana, 1983); Atlantic cod (Gadus morhua) (Fleming, 1960); and American plaice (Hippoglossoides platessoides) (Walsh, 1994). As well, intra-population changes in maturation over time have been demonstrated for Atlantic cod in the northeast Arctic (Jorgensen, 1990) on the Scotian Shelf (Beacham, 1983) and on the Flemish Cap (Saborido-Rey and Junquera, 1999), as well as for North Sea plaice (Pleuronectes platessa) (Rijnsdorp, 1989), witch flounder (Glyptocephalus cynoglossus) (Bowering, 1989) and American plaice (Pitt, 1975; Bowering and Brodie, 1991; Morgan and Colbourne, 1999).
American plaice populations from the waters off the east and south coasts of Newfoundland and Labrador provide an excellent example of both inter and intra population variation in maturity (Fig. 1). Maturity at age and size were estimated by cohort using generalized linear models with a logit link function and binomial error (Morgan and Colbourne, 1999). These three populations show a decline in both age and size at maturity as population abundance declined. Inter-population differences are also evident, in particular American plaice in SubDiv. 3Ps in recent cohorts mature at an older age and larger size than those in the other two populations and the rate of decline in length at 50% maturity was less for this population. The increase in length at maturity in Div. 2J3K and Div. 3LNO in the recent part of the time series, with no increase in age at maturity is reflective of increased growth (i.e. increased length-at-age) that has not been associated with maturity at an older age
Changes in maturity schedules at age and length have been linked with changes in abundance in numerous studies. Increased resources, available to individuals at low population size, results in an increase in growth rate (i.e. density dependent growth response) and is thought to result in maturation at a younger age (Pitt, 1975; Bowering, 1989; Jorgensen, 1990; Rijnsdorp, 1993). Higher temperature has also been shown to lead to earlier maturity through increased growth (Alm, 1957; Sandström et al., 1995). Related to growth are changes in condition, with fish in better condition more likely to mature younger and smaller (Marteinsdottir and Begg, 2002; Morgan, 2004; Morgan and Lilly, 2006; Grift et al., 2007). Changes in mortality have also been identified as a cause of variation in maturity in fishes. Increased mortality can select for maturation at a younger age (Diana, 1983; Roff, 1992; Hutchings, 1993; Fox, 1994; Olsen et al., 2005) and smaller size (Kasperski and Kozlowski, 1993). This is because those that delay maturation have a decreased probability of surviving to reproduce when mortality increases. The level of adult mortality relative to juvenile mortality can also affect age and size at maturity (Gadgil and Bossert, 1970; Hutchings, 1993).
The data that exist on maturity can easily be incorporated into stock assessment by including their estimates in the calculation of spawning stock biomass. Historically estimates of SSB have assumed that all fish mature at the same age (referred to as knife edge maturity) or use a single invariant maturity ogive.
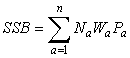 
where Na is the number of fish at age a, Wa is the weight of fish at age a, and Pa is the proportion mature at age a. Changes in maturity at age are incorporated by estimating Pa, preferably for each cohort. Often the proportion mature used is the female only ogive.
Jakobsen (1992) found that using cohort estimates of maturity for northeast Arctic cod resulted in a higher Fmed (the fishing mortality (F) which results in RP per recruit (R) equal to the median of the observed R per RP) than using a knife edge estimate of maturity of 8+. Incorporation of variable maturity, estimated by cohort, can result in substantial differences in perceived productivity (Morgan and Brattey, 2005). Model estimates of maturity were found to improve the relationship between estimates of potential egg production and estimates of egg production from egg surveys in Baltic cod (Kraus et al., 2002).
The incorporation of maturity estimates into the estimation of limit reference points is illustrated here using American plaice in NAFO Div. 3LNO (the Grand Bank). Data on population number and weights-at-age come from Dwyer et al. (MS 2005). Two indices of RP were produced: 9+ biomass (used as a proxy for SSB for this population prior to the application of estimates of maturity at age), and SSB (see equation above). Example reference points were calculated for each index of RP with Bref being the change point in a segmented regression of recruits against RP and the fishing mortality reference point being Fmed.
The relationship between RP and recruitment is different for the two indices of RP, resulting in substantially different reference points (Fig. 2). Bref was 179 000 t when 9+ biomass was the index of RP and 98 000 t when SSB was used. Fmed was 0.19 for 9+ biomass and 0.44 for SSB.
Sex ratio
Although data on sex ratio are generally as widely available as those on maturity, as they are collected as part of the process of sampling for maturity, they have not received as much attention. As with maturity, there can be differences in sex ratio between populations of the same species as well as temporal trends within populations (Rijnsdorp, 1994; Hunt, 1996; Marshall et al., 1998; Jakobsen and Ajiad, 1999; Kraus et al., 2002; Morgan and Brattey, 2005). Again this can be illustrated using data for American plaice around Newfoundland (Fig. 3). Here sex ratio was estimated for each population with a generalized linear model with a logit link function and a binomial error. Both age and cohort were treated as class (categorical) variables (Morgan and Brattey, 2005). All three populations show an increase in proportion female for cohorts of the mid 1980s. For Div. 3LNO and SubDiv. 3Ps American plaice, this higher proportion female continues to about the mid 1990s, while sex ratio declined for Div. 2J3K for the 1990 cohort. However, 3Ps exhibits much more variability from cohort to cohort and has a slightly lower proportion female at the oldest age compared to 3LNO or 2J3K. Differences in sex ratio between cohorts could be a result of differential spawning mortality between the sexes and/or changes in fishing mortality on the sexes (Jakobsen and Ajiad, 1999).
Sex ratio can be added to estimates of RP to produce female spawning biomass:
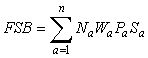 
where Sa is the proportion female at age a. Pa is the proportion mature at age a for females and Wa is weight at age which may be sex specific but is often for males and females combined.
Morgan and Brattey (2005) found that incorporation of sex ratio resulted in differences in perceived productivity for three cod stocks. The use of estimates of sex ratio improved the relationship between estimates of egg production from egg surveys and estimates of potential egg production in Baltic cod. The effect of sex ratio on improving estimates of potential egg production was less than the effect of maturity (Kraus et al., 2002). The addition of estimates of sex ratio at length to SSB to produce FSB for northeast Arctic cod produced some difference in the perception of stock status relative to Blim (Marshall et al., 2006). Blim is the RP below which the stock should not be allowed to fall, in some cases it is considered the level of RP below which R is impaired.
Sex ratio was incorporated into estimates of RP for American plaice to produce FSB using the equation given above. Again Fmed and Bref were calculated in the same manner as for SSB and 9+ biomass. The estimate of Fmed of 0.35 (Fig. 2) was substantially different than the estimate for 9+ biomass (0.19), but differed somewhat less from that produced from using SSB as the index of RP (0.44). Bref was 111 000 t, similar to that derived using SSB as the estimate of RP, but almost 70 000 t lower than that derived when using 9+ biomass.
Fecundity
Data on fish fecundity are much less available than data on maturity and sex ratio (Tomkiewicz et al., 2003). Variation in fecundity between populations of the same species and between years within a population is well known (Bowering, 1978; Pinhorn, 1984; Gundersen et al., 1999; Kraus et al., 2002; Blanchard et al., 2003; Power et al., 2005; Rideout and Morgan, 2007).
American plaice have some fecundity data that can be used as an example of variability in fecundity. Fecundity length relationships were fit for Div. 3LNO and SubDiv. 3Ps American plaice, from 1993 to 1996. From these relationships the number of eggs produced by a 40 cm female was calculated for each year. There are population differences and variation over the short time series that is available (Fig. 4).
Factors such as nutritional condition or ration level have been found to be positively related to fecundity (Kjesbu et al., 1991; Millner et al., 1991; Rijnsdorp, 1991; Rijnsdorp et al., 1991; Kjesbu et al.,1998; Ma et al., 1998; Lambert and Dutil, 2000). Selective pressures from fisheries have also been suggested as a factor in changes in fecundity with fishing mortality selecting for fish that have a higher investment in reproduction earlier in life, including increased fecundity (Yoneda and Wright, 2004; Wright, MS 2005).
Fecundity data can be incorporated into indices of RP in a similar fashion to the previously discussed factors such that:
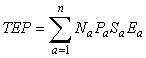 
where TEP is total egg production, Ea is the number of eggs produced at age, usually from a fecundity length or fecundity weight relationship applied to length or weight at age.
A constant fecundity length relationship used to produce TEP for Georges Bank cod produced little difference in percent maximum spawning potential at different levels of F compared to using SSB as the index of RP. This may have been because there was little variation in mean length-at-age in the time series examined (Murawski et al., 2001). Morgan and Brattey (2005) also used constant fecundity length relationships to produce time series of TEP for three cod populations. Perceived productivity of the populations differed for TEP as compared to FSB, SSB and estimates of RP using knife edge maturity. Given the scarcity of fecundity data, proxies for fecundity have been suggested as an alternative approach (see Lambert et al. (2003) for a review of potential proxies). Marshall et al. (2006) used the relationship between fecundity, length and condition to produce a time series of annual egg production estimates for northeast Arctic cod. This resulted in an index of RP that gave a different estimate of Blim and perception of stock status relative to Blim, than SSB or FSB. Kraus et al. (2002) used the relationship between relative fecundity and prey availability to produce a time series of relative fecundity for Baltic cod. This gave an improved S/R relationship (a higher r2 in a linear regression with recruitment) and better relationship between estimates of potential egg production and results of egg surveys for Baltic cod. However, DeOliveira et al. (2006) in simulation studies with horse mackerel (Trachurus trachurus) assuming a constant proportion harvesting strategy, found that unless there was a well estimated proxy with a strong relationship between the proxy and fecundity, the population fell below the biological reference point more often than if constant fecundity was assumed.
American plaice in Div. 3LNO is again used as an example, with TEP being calculated by applying a time invariant fecundity length relationship to mean length at age. Fmed using TEP is 0.36, similar to that derived using FSB as the index of RP, but differing substantially from Fmed for SSB and 9+ biomass (Fig. 5). Because of differences in scale (eggs×1012 vs thousands of tons of biomass) Bref can not be directly compared with the biomass based indices of RP. However, the number of years in which the index of RP is below the Bref derived using that index can be compared to determine whether the different indices of RP result in different perceptions about stock status. For 9+ biomass the population was below Bref for 33 years, for SSB 23 years, for FSB 35 years and for TEP the population was below Bref for 34 years of the time series.
The difference in estimated reference points is a result of different relationships with recruitment for each estimate of RP. It is also a function of the method chosen to estimate the reference point. In these examples the estimate of RP that incorporates the least reproductive biology, 9+ biomass, gives the lowest estimate of Fmed. However, if Fcrash (F resulting in a high probability of population collapse, derived either from a production model or S/R curve) is calculated for SSB and 9+ biomass, the estimate is lower for SSB (0.49) than for 9+ biomass (0.55). Another important factor to consider is how well the reference points are estimated. For this example, the coefficient of variation for the estimate of Bref was lower for the two indices of RP that incorporated the most reproductive biology (9+ biomass CV=33.1, SSB CV=33.3, FSB CV=26.4, TEP CV=27.2), indicating statistically better estimates of the reference point. For each population, the best method for estimating reference points and the best way to estimate RP must be determined during the assessment process. The degree to which perceptions of stock productivity differ with different indices of RP will vary with population, depending on how much variation there has been in maturity, sex ratio and fecundity and how this variation affects the S/R relationship. However, from these examples, and the work of others cited above, it is clear that differing indices of RP can have a large impact on estimates of reference points and perceptions of stock status.
Skipped spawning
The failure to spawn on an annual basis has been demonstrated in numerous fish species (see Rideout et al. (2005a) for a review). Fish that make the decision not to spawn may fail to develop eggs or may produce eggs but retain or resorb them (atresia). Spawning omission has been found to be caused by a variety of factors including high or low population density (possibly detected by individuals by pheromones) and temperature (Swingle, 1954; Hodder, 1965; Fedorov, 1971; Dahlgren, 1979; Stacey, 1984; Trippel and Harvey, 1990; Pörtner et al., 2001; Stacey and Sorensen, 1991). By far the most commonly reported cause of skipped spawning is low condition (Hislop et al., 1978; Burton and Idler, 1987; Rijnsdorp, 1990; Oganesyan, MS 1993; Maddock and Burton, 1994; Rideout et al., 2000). However, there is evidence that the probability of spawning omission at a given level of condition may be affected by diet composition (Rideout et al., 2006; Rideout and Rose, 2006). In addition, fish may resorb only some oocytes, rather than totally skipping spawning (Hislop et al., 1978; Ma et al., 1998; Thorsen et al., 2006).
A large and varying proportion of skipped spawning fish could obviously result in a poor estimate of RP if not taken into account. Where sufficient data exist, estimates of skipped spawning can be applied to estimates of RP in a similar manner as maturity, sex ratio and fecundity, producing an index of RP that is reduced by the proportion failing to spawn. However, data are rarely available to produce a time series of RP excluding fish which will fail to spawn. Burton (1999) applied a range of estimates of the proportion of skipped spawning individuals taken from other populations to the SSB for Pacific halibut (Hippoglossus hippoglossus) and North Sea plaice (Pleuronectes platessa). She found an improvement in the S/R relationship for RP incorporating estimates of skipped spawning. Failing to account for the varying proportion of skipped spawning fish at length in a group of inshore cod, was found to result in the overestimation of egg production by between 8–41% over a period of six years (Rideout and Rose, 2006). Estimating the number of spawning individuals based on the relationship between condition and the probability of spawning gave estimates that indicated a relatively high level of spawning omission in each year ranging from 8–30% for SubDiv. 3Ps cod (Morgan and Rideout, MS 2005). However, excluding skipped spawning fish from the SSB did not improve the S/R relationship.
Egg and larval viability
Reproductive potential will also be influenced by changes in egg and larval viability. There is evidence that egg and larval quality (or their correlate egg size) is affected by female size and condition, with larger, better conditioned females producing higher quality eggs and larvae. Egg size may also vary over the spawning season in fish that spawn multiple batches (Chambers and Waiwood, 1996; DeMartini, 1991; Kjesbu et al., 1996; Marteinsdottir and Steinarsson, 1998; Rideout et al., 2005b). There is also some evidence that repeat and/or older spawners may produce more viable eggs and larvae than first time spawners (Solemdal et al., MS 1992; Kjesbu et al., 1996; Trippel, 1998; Vallin and Nissling, 2000; Berkeley et al., 2004).
This is probably the least well studied of the factors discussed so far. Problems with conducting experiments on large, long lived species, as well as the difficulty in collecting information on factors such as fertilization and hatching success in the wild have limited the availability of data on egg and larval quality. Developing quantitative measures of egg and larval viability to add to the equations of RP is likely to prove difficult. However, studies generally indicate that a spawning stock composed of larger (older) females in better condition may have a higher RP through the production of more viable eggs and larvae. Deviations of RP from that calculated not taking egg and larval viability into account were found to be greater at increased fishing mortality (O’Farrell and Botsford, 2006; Scott et al., 2006). Vallin and Nissling (2000) found a clear relationship between recruitment and egg production by older Baltic cod which produce eggs that are more viable because they are larger with neutral buoyancy at a lower salinity. Murawski et al. (2001) included assumptions about egg and larval viability as an extension to the standard equations used to calculate spawner per recruit (SPR). They then used these alternative indices of SPR to estimate fishing mortality reference points. Fcrash was lower when the metric of RP was the number of viable larvae produced (modelled as a function of egg diameter and length and of spawning experience) rather than SSB. Spencer et al. (2007) incorporated a decrease in viable larvae with increasing age in the calculation of the F which resulted in the conservation of a specific percentage of reproductive potential per recruit relative to an unfished population for Alaska Pacific ocean perch (Sebastes alutus). They found that incorporating larval viability estimates led to lower F reference points. These studies indicate that not incorporating variation in egg and larval viability can lead to over estimation of RP and potentially over estimation of sustainable fishing mortality.
Other Uses of Reproductive Biology in Management Advice
The examples given above mainly deal with the role of reproductive biology in calculating reproductive potential from the results of traditional age structured population models. Estimation of reproductive characteristics is also an essential aspect of egg production methods. These methods use ichthyoplankton surveys to determine annual egg production. To calculate SSB from these surveys, good estimates of fecundity, atresia, and sex ratio are required (Armstrong et al., 2001; MS 2007).
While the main role for information on reproductive biology in fisheries science is probably the provision of advice on sustainable levels of F, such information can also be used to provide advice on other management measures. For example, spawning areas and times can be mapped to provide advice on closed areas and seasons. This can apply to fishing but also to other activities such as seismic surveying. A variety of human activities can have a potential impact on population productivity (e.g. Sandström et al., 1995) and so studies on the causes and consequences of changes in productivity can provide important information for managers in a wide range of situations. Information on size at maturity can be used to inform minimum landing size regulations. Changes in reproductive biology, such as maturity-at-age, have been suggested as a means of detecting harm from fisheries, perhaps serving as an early warning signal (Trippel, 1995; Olsenet al., 2005).
Future Directions
One of the main areas that needs to be addressed in the integration of RP into fisheries advice is whether or not its incorporation results in an improvement in that advice. Much of the research on the RP of commercial fish species has been motivated by the poor fit of S/R models to the available data. The rationale being that SSB is not a good estimate of RP and that, by incorporating variables which move us closer to viable egg and larval production, we will improve these estimates. Yet few studies actually test for an improved S/R relationship or improved ability to predict recruitment (for some exceptions see Marteinsdottir and Thorarinsson, 1998; Murawski et al., 2001; Marshall et al., 2006). One way to do this is through cross validation. Each S/R pair is omitted in turn, the S/R model refit and the omitted R predicted. The index of RP giving the lowest residual sums of squares from this procedure should provide a better prediction of recruitment. As an illustration, Ricker S/R curves were fit to the four indices of RP for American plaice described above. It is clear that there is not a large difference in the model fit (Fig. 6). The Akaike information criteria for the fits are: 9+ biomass 45.5; SSB 59.1; FSB 53.2; TEP 53.2. The lowest cross validation residual sums of squares was actually for 9+ biomass. However, SSB, FSB and TEP all predict more recent (last 8 years) recruitment better than 9+ biomass. This is an important consideration for short or medium term projections. Marshall et al. (2006) and Murawski et al. (2001) both assessed model fit for various indices of RP. Marshall et al. (2006) found that SSB gave better model fit (lower residual sums of squares and higher r2) than FSB or TEP while Murawski et al. (2001) found better model fit (lower residual sums of squares) using viable larvae rather than SSB.
Another aspect of ‘performing better’ is how robust is the advice generated using different indices of RP and how sensitive is it to the different assumptions that are incorporated. Is the risk of the stock being outside safe limits lower using a particular index of RP? The simulation study by DeOliveira et al. (2006) on constant fecundity versus proxies for fecundity provides a good example. Simulations of this type would be very helpful in establishing whether more complex indices of RP perform better for particular stocks.
The results of such exercises evaluating the performance of different indices of RP will likely indicate that the ‘optimum’ index of RP will vary from stock to stock. This will depend on the precision and accuracy of the various estimates being incorporated. If additional biological parameters are poorly estimated some stocks may achieve better performance using only constant assumptions about maturity, sex ratios and other reproductive characteristics.
Another area that requires research is the choice of S/R curve. There are a number of standard S/R curves that are applied to S/R data in stock assessments (Needle, 2002). However, often the S/R data do not conform to any of these functional shapes. As Needle (2002) points out, there has been little change in the S/R methods used by the majority of fisheries scientists in 50 years and we may benefit from applying non-traditional approaches. Using a model that is based on some theoretical relationship between RP and R is perhaps ideal, but using a model that obviously does not describe the underlying data is not going to result in a good prediction of R. There should be investigation of alternative functional forms and of the use of a variety of non-parametric smoothers to describe these data.
Further research should also be conducted into the methods used to estimate reproductive characteristics. Maturities are usually estimated by either age or size, but both factors can affect the maturation process and attempts should be made to model maturity as a function of both (for example see Korsbrekke, 1999). Another example is the proper model to estimate sex ratio. In the example used here for American plaice, both cohort and age were class variables. This means that every cohort has the same estimated age effects. Clearly there could be variation in the age effect between cohorts, and alternative modelling approaches should be investigated. Sampling effects should also be considered. Often sampling programs have been established for many years without analyses of the appropriate sample size or geographic distribution of sampling. New sampling techniques that simplify or speed the sampling process, such as recent developments in estimating fecundity (Thorsen and Kjesbu, 2001; Friedland et al., 2005) or which enhance our ability to accurately sample biological characteristics, should continue to be an area of research. Our ability to estimate reproductive characteristics is still limited in many cases by a lack of data. Efforts should continue toward increasing the number of populations/species for which adequate data exist to develop improved indices of RP.
Many commercial fish stocks are currently in a depleted state. They have under gone changes in their RP as stock size declined. For those stocks that recover, changes in reproductive parameters should be closely monitored. The study of Georges Bank herring (Melvin and Stephenson, 2006) provides a rare example. If more stocks can be studied during rebuilding, it might provide useful insight into such issues as timing of changes in RP relative to changes in stock abundance and the ability of populations to return to pre-collapse values of their reproductive characteristics.
Factors determining changes in reproductive biology are varied. While many of the causes of change are known, often much variability remains to be explained. Interactions between forces acting on reproductive investment are often only generally known and not quantitatively described. In addition, individual decisions regarding such things as maturation, growth and fecundity are inter-related and involve tradeoffs between reproduction, growth and survival. These in turn can impact mortality and/or be affected by changes in mortality. However, while theory exists on how tradeoffs between these life history traits are made, there are few studies which have tried to quantify these interactions at a level that would be useful for improving our predictions about changes in RP. Further empirical and experimental studies on the factors affecting reproductive characteristics and the interactions between them, would be of great benefit. In addition, modelling exercises can help to shed light on these tradeoffs. Two examples of studies attempting to do this are companion papers by Jørgensen and colleagues. In these papers, tradeoffs in energy allocation are examined in a life history context to explore optimal decisions regarding growth, maturation, fecundity and skipped spawning for Northeast Arctic cod (Jørgensen and Fiksen, 2006; Jørgensen et al., 2006). They use dynamic programming to find the optimal solutions to these tradeoffs. The models are realistic but complex and require a substantial amount of paramaterization. The degree of information required for such a modelling exercise would be available for very few populations. Nonetheless, the approach is an example of integrating the many complex tradeoffs involved in life history decisions. Studies which attempt to understand the suite of decisions made by individuals faced with sometimes opposing forces will be essential in building better predictions of how reproductive biology will respond to natural or man made changes.
Conclusion
Reproductive biology is being integrated into advice for fisheries management mainly through the production of alternative indices of RP. These alternative indices can then be used in the same way as more traditional measures; in estimates of stock status, setting of limit reference points and projections of stock trajectory under different management options. There are many stock assessments which already include at least some reproductive biology in the formulation of advice (Marshall et al., 2003). A few examples are 3M cod (Murua et al., MS 2006), 3LNO American plaice (Dwyer et al., MS 2005), 3Ps cod (Brattey et al., MS 2005), 3Pn4RS cod (Fréchet et al., MS 2005) Georges Bank cod (O’Brien et al., 2006), northeast Arctic cod (Anon., MS 2007a) and Baltic cod (Anon., MS 2007b). These are assessments from NAFO, Canada, the USA and ICES; there are many others.
The examples considered in this paper show clearly that alternative indices of RP can lead to different perceptions of productivity, limit reference points and stock status. However, it is not always clear which index of RP is the best one for a particular population. If there has been little variation in reproductive characteristics over time, then there will be little difference in the results using various indices of RP. If reproductive characteristics are poorly estimated then simpler indices of RP may perform better. To determine which index of RP performs best for a particular population the best S/R relationship for each index should be used, not simply the same S/R function for all as was used here for comparative purposes. In addition, this area would benefit from simulations evaluating the performance of different indices of RP in maintaining populations within safe biological limits. In such evaluations RP specific S/R relationships should be used and the reference points should also be derived from the RP index being evaluated. There has been little work in this area.
The scope for integrating reproductive biology into fisheries advice is expanding. There is increased use of management strategy evaluation which should be based on as realistic a model of population biology as possible. Recovery potential evaluation requires good estimates of current productivity and how that might change in the near future. Studies of reproductive biology are also integral to any ecosystem approach to fisheries management. Human activities of various kinds can alter population productivity and these changes in productivity can have effects on all components of the ecosystem. During this process of integration of reproductive biology into advice for fisheries management, much has been learned about the reproductive biology of fishes. There is still much to learn. Improving our ability to predict how life history decisions will change under differing conditions would be a major advance. Further progress in the integration of reproductive biology into fisheries management advice also depends on clearly demonstrating the benefits of using the optimum index of RP for a stock in estimates of productivity.
Acknowledgements
This manuscript was generated from discussions and activities of the Northwest Atlantic Fisheries Organization's Working Group on Reproductive Potential. I thank Peter Shelton for many helpful comments and discussions on the manuscript. Rick Rideout and two anonymous reviewers gave helpful comments on an earlier version of the MS. I thank the many people involved in the collection of data on American plaice used here. The COST action Fish Reproduction and Fisheries (FRESH) supported the presentation of this paper at the NAFO/PISCES/ICES symposium on Reproductive and Recruitment Processes of Exploited Marine Fish Stocks.
References
ALM, G. 1957. Connections between maturity, size and age in fishes. Rep. Inst. of Freshwater Res., Drottningholm, 40: 5–145.
ANONYMOUS. MS 2007a. Report of the Arctic fisheries working group (AFWG). ICES CM 2007/ACFM:16, 661 p.
MS 2007b. Report of the Baltic fisheries assessment working group (WGBFAS). ICES CM 2007/ACFM:15, 746 p.
ARMSTRONG, M. J., P. CONNOLLY, R. D. M. NASH, M. G. PAWSON, E. ALESWORTH, P. J. COULAHAN, M. DICKEY-COLLAS, S. P. MILLIGAN, M. O'NEILL, P. R. WITTHAMES, and L. WOOLNER. 2001. An application of the annual egg production method to estimate spawning biomass of cod (Gadus morhua L.), plaice (Pleuronectes platessa L.) and sole (Solea solea L.) in the Irish Sea. ICES J. Mar. Sci., 58: 183–203. doi:10.1006/jmsc.2000.1001
ARMSTRONG, M., F. GOODSIR, L. GREENWOOD, S. MULLIGAN, N. TAYLOR, P. WITTHAMES, C. FOX, A. PRAEL, P-J. SCHÖN, and H. GERRITSEN. MS 2007. Developing an egg-survey approach to monitoring the biomass of cod and other demersal fish species in the Irish Sea. ICES CM 2007/Q:25.
BEACHAM, T. D. 1983. Variability in median size and age at sexual maturity of Atlantic cod, Gadus morhua, on the Scotian shelf in the northwest Atlantic ocean. Fish. Bull., 81: 303–321.
BELK, M. C. 1995. Variation in growth and age at maturity in bluegill sunfish: genetic or environmental effects? J. Fish Biol., 47: 237–247. doi:10.1111/j.1095-8649.1995.tb01891.x
BERKELEY, S. A., C. CHAPMAN, and S. M. SOGARD. 2004. Maternal age as a determinant of larval growth and survival in a marine fish, Sebastes melanops. Ecol., 85: 1258–1264. doi:10.1890/03-0706
BLANCHARD, J. L., K. T .FRANK, and J. E. SIMON. 2003. Effects of condition on fecundity and total egg production of eastern Scotian Shelf haddock. Can. J. Fish. Aquat. Sci., 60: 321–332. doi:10.1139/f03-024
BOWERING, W. R. 1978. Fecundity of witch flounder (Glyptocephalus cynoglossus) from St. Pierre Bank and the Grand Bank of Newfoundland. J. Fish. Res. Board Can., 35: 1199–1206.
1989. Witch flounder distribution off southern Newfoundland, and changes in age, growth, and sexual maturity patterns with commercial exploitation. Trans. Amer. Fish. Soc., 118: 659–669. doi:10.1577/1548-8659(1989)118<0659:WFDOSN>2.3.CO;2
BOWERING, W. R., and W. B. BRODIE. 1991. Distribution of commercial flatfishes in the Newfoundland-Labrador region of the Canadian northwest Atlantic and changes in certain biological parameters since exploitation. Neth. J. Sea Res., 27: 407–422. doi:10.1016/0077-7579(91)90042-Y
BRATTEY, J., N. G. CADIGAN, B. P. HEALEY, G. R. LILLY, E. F. MURPHY, P. A. SHELTON, and J. C. MAHÉ. MS 2005. An assessment of the cod (Gadus morhua) stock in NAFO Subdiv. 3Ps in October 2005. Can. Sci. Adv. Sec. Res. Doc., 2005/07, 106 p.
BURTON, M. P. M. 1999. Notes on potential errors in estimating spawning stock biomass: determining the effects of non-participating adults for some groundfish species. J. Northw. Atl. Fish. Sci., 25: 205–213. doi:10.2960/J.v25.a18
BURTON, M. P., and D. R. IDLER. 1987. An experimental investigation of the non-reproductive, post-mature state in winter flounder. J. Fish Biol., 30: 643–650. doi:10.1111/j.1095-8649.1987.tb05793.x
CHAMBERS, R. C., and K. G. WAIWOOD. 1996. Maternal and seasonal differences in egg sizes and spawning characteristics of captive Atlantic cod, Gadus morhua. Can. J. Fish. Aquat. Sci., 53: 1986–2003.
DAHLGREN, B. T. 1979. The effects of population density on fecundity and fertility in the guppy, Poecilia reticulata (Peters). J. Fish Biol., 15: 71–91. doi:10.1111/j.1095-8649.1979.tb03573.x
DEMARTINI, E. E. 1991. Annual variations in fecundity, egg size and the gonadal and somatic condition of queenfish Seriphus politus (Sciaenidae). Fish. Bull., 89: 9–18.
DEOLIVEIRA, J. A. A., B. A. ROEL, and M. DICKEY-COLLAS. 2006. Investigating the use of proxies for fecundity to improve management advice for western horse mackerel Trachurus trachurus. ICES J. Mar. Sci., 63: 25–35. doi:10.1016/j.icesjms.2005.07.006
DIANA, J. S. 1983. Growth, maturation, and production of northern pike in three Michigan lakes. Trans. Amer. Fish. Soc., 112: 38–46. doi:10.1577/1548-8659(1983)112<38:GMAPON>2.0.CO;2
DWYER, K. S., M. J. MORGAN, D. MADDOCK PARSONS, W. B. BRODIE, B. P. HEALEY, P. A. SHELTON, and H. MURUA. MS 2005. An assessment of American plaice in NAFO Divisions 3LNO. NAFO SCR Doc., No. 61, Serial No. N5147, 79 p.
FEDOROV, K. Y. 1971. The state of the gonads of the Barents Sea Greenland halibut (Reinhardtius hippoglossoides (Walb.)) in connection with failure to spawn. J. Ichthyol., 11: 673–682.
FLEMING, A. M. 1960. Age, growth and sexual maturity of cod (Gadus morhua L.) in the Newfoundland area, 1947-1950. J. Fish. Res. Board Can., 17: 775–809.
FOX, M. G. 1994. Growth, density, and interspecific influences on pumpkinseed sunfish life histories. Ecol., 75: 1157–1171. doi:10.2307/1939439
FRÉCHET, A., J. GAUTHIER, P. SCHWAB, L. PAGEAU, C. SAVENKOFF, M. CASTONGUAY, D. CHABOT, C. TOURNOIS, J-F. LUSSIER, J. SPINGLE, and F. COLLIER. MS 2005. The status of cod in the northern Gulf of St. Lawrence (3Pn, 4RS) in 2004. Can. Sci. Adv. Sec. Res. Doc., 2005/60, 75 p.
FRIEDLAND, K. D., D. AMA-ABASI, M. MANNING, L. CLARKE, G. KLIGYS, and R. C. CHAMBERS. 2005. Automated egg counting and sizing from scanned images: rapid sample processing and large data volumes for fecundity estimates. J. Sea Res., 54: 307–316. doi:10.1016/j.seares.2005.06.002
GADGIL, M., and W. H. BOSSERT. 1970. Life historical consequences of natural selection. Am. Nat., 104: 1–24. doi:10.1086/282637
GRIFT, R. E., M. HEINO, A. D. RIJNSDORP, S. B. M. KRAAK, and U. DIECKMANN. 2007.Three-dimensional maturation reaction norms for North Sea plaice. Mar. Ecol. Prog. Ser., 334: 213–224. doi:10.3354/meps334213
GUNDERSEN, A. C., O. S. KJESBU, K. H. NEDREAAS, and A. STENE. 1999. Fecundity of northeast Arctic Greenland halibut (Reinhardtius hippoglossoides). J. Northw. Atl. Fish. Sci., 25: 29–36. doi:10.2960/J.v25.a3
HILBORN, R., and C. J. WALTERS. 1992. Quantitative fisheries stock assessment: choice, dynamics and uncertainty. Chapman and Hall, New York, 570 p.
HISLOP, J. R. G., A. P. ROBB, and J. A. GAULD. 1978. Observations on effects of feeding level on growth and reproduction in haddock, Melanogrammus aeglefinus (L.) in captivity. J. Fish Biol., 13: 85–98. doi:10.1111/j.1095-8649.1978.tb03416.x
HODDER, V. M. 1965. The possible effects of temperature on the fecundity of Grand Bank haddock. ICNAF Spec. Pub., 6: 515–522.
HUNT, J. J. 1996. Rates of sexual maturation of Atlantic cod in NAFO Division 5Ze and commercial fishery implications. J. Northw. Atl. Fish. Sci., 18: 61–75.
HUTCHINGS, J. A. 1993. Adaptive life histories effected by age-specific survival and growth rate. Ecol., 74: 673–684. doi:10.2307/1940795
JAKOBSEN, T. 1992. Biological reference points for North-East Arctic cod and haddock. ICES J. Mar. Sci., 49: 155–166. doi:10.1093/icesjms/49.2.155
JAKOBSEN, T., and A. AJIAD. 1999. Management implications of sexual differences in maturation and spawning mortality of Northeast Arctic cod. J. Northw. Atl. Fish. Sci., 25: 125–131. doi:10.2960/J.v25.a11
JØRGENSEN, C., and Ø. FIKSEN. 2006. State-dependent energy allocation in cod (Gadus morhua). Can. J. Fish. Aquat. Sci., 63: 186–199. doi:10.1139/f05-209
JØRGENSEN, C., B. ERNANDE, Ø. FIKSEN, and U. DIECKMANN. 2006. The logic of skip spawning in fish. Can. J. Fish. Aquat. Sci., 63: 200–211. doi:10.1139/f05-210
JØRGENSEN, T. 1990. Long-term changes in age at sexual maturity of northeast Arctic cod (Gadus morhua L.). J. Cons. Perm. Inter. Explor. Mer, 46: 235–248.
KASPERSKI, W., and J. KOZLOWSKI. 1993. The effect of exploitation on size at maturity in laboratory populations of guppies Poecilia reticulata (Peters). Acta Hydrobiol., 35: 65–72.
KJESBU, O. S., J. KLUNGSØYR, H. KRYVI, P. R. WITTHAMES, and M. GREER WALKER. 1991. Fecundity, atresia, and egg size of captive Atlantic cod (Gadus morhua) in relation to proximate body composition. Can. J. Fish. Aquat. Sci., 48: 2333–2343. doi:10.1139/f91-274
KJESBU, O. S., P. SOLEMDAL, P. BRATLAND, and M. FONN. 1996. Variation in annual egg production in individual captive Atlantic cod (Gadus morhua). Can. J. Fish. Aquat. Sci., 53: 610–620. doi:10.1139/cjfas-53-3-610
KJESBU, O. S., P. R.WITTHAMES, P. SOLEMDAL, and M. GREER WALKER. 1998. Temporal variations in the fecundity of Arcto-Norwegian cod (Gadus morhua) in response to natural changes in food and temperature. J. Sea Res., 40: 303–321. doi:10.1016/S1385-1101(98)00029-X
KORSBREKKE, K. 1999. Variations in maturity of haddock, in the Barents Sea in relation to year-class strength, age, size, sex and area. J. Northw. Atl. Fish. Sci., 25: 37–45. doi:10.2960/J.v25.a4
KRAUS, G., J. TOMKIEWICZ, and F. W. KÖSTER. 2002. Egg production of Baltic cod (Gadus morhua) in relation to variable sex ratio, maturity, and fecundity. Can. J. Fish. Aquat. Sci., 59: 1908–1920. doi:10.1139/f02-159
LAMBERT, Y., and J. D. DUTIL. 2000. Energetic consequences of reproduction in Atlantic cod (Gadus morhua) in relation to spawning level of somatic energy reserves. Can. J. Fish. Aquat. Sci., 57: 815–825. doi:10.1139/cjfas-57-4-815
LAMBERT, Y., N.A. YARAGINA, G. KRAUS, G. MARTEINSDOTTIR, and P. J. WRIGHT. 2003. Using environmental and biological indices as proxies for egg and larval production of marine fish. J. Northw. Atl. Fish. Sci., 33: 115–159. doi:10.2960/J.v33.a7
MA, Y., O. S. KJESBU, and T. JØRGENSEN. 1998. Effects of ration on the maturation and fecundity in captive Atlantic herring (Clupea harengus). Can. J. Fish. Aquat. Sci., 55: 900–908. doi:10.1139/cjfas-55-4-900
MADDOCK, D. M., and M. P. M. BURTON. 1994. Some effects of starvation on the lipid and skeletal muscle layers of the winter flounder, Pleuronectes americanus. Can. J. Zool., 72: 1672–1679.
MARSHALL, C. T., O. S. KJESBU, N. A. YARAGINA, P. SOLEMDAL, and O. ULLTANG. 1998. Is spawner biomass a sensitive measure of the reproductive and recruitment potential of northeast Arctic cod? Can. J. Fish. Aquat. Sci., 55: 1766–1783. doi:10.1139/cjfas-55-7-1766
MARSHALL, C. T., L. O'BRIEN, J. TOMKIEWICZ, G. MARTEINSDÓTTIR, M. J. MORGAN, F. SABORIDO-REY, F. KÖSTER, J. L. BLANCHARD, D. H. SECOR, G. KRAUS, P. WRIGHT, N. V. MUKHINA, and H. BJÖRNSSON. 2003. Developing alternative indices of reproductive potential for use in fisheries management: case studies for stocks spanning an information gradient. J. Northw. Atl. Fish. Sci., 33:161–190. doi:10.2960/J.v33.a8
MARSHALL, C. T., C. L. NEEDLE, A. THORSEN, O. S. KJESBU, and N. A. YARAGINA. 2006. Systematic bias in estimates of reproductive potential of an Atlantic cod (Gadus morhua) stock: implications for stock-recruit theory and management. Can. J. Fish. Aquat. Sci., 63: 980–994. doi:10.1139/F05-270
MARTEINSDOTTIR, G., and G. A. BEGG. 2002. Essential relationships incorporating the influence of age, size and condition on variables required for estimation of reproductive potential in Atlantic cod Gadus morhua. Mar. Ecol. Prog. Ser., 235: 235–256. doi:10.3354/meps235235
MARTEINSDOTTIR, G., and A. STEINARSSON. 1998. Maternal influences on the size and viability of Iceland cod Gadus morhua eggs and larvae. J. Fish Biol., 52: 1241–1258.
MARTEINSDOTTIR, G., and K. THORARINSSON. 1998. Improving the stock-recruitment relationships in Icelandic cod (Gadus morhua) by including age diversity of spawners. Can. J. Fish. Aquat. Sci., 55: 1372–1377. doi:10.1139/cjfas-55-6-1372
MELVIN, G. D., and R. L. STEPHENSON. 2007. The dynamics of a recovering fish stock: Georges Bank herring. ICES J. Mar. Sci., 64: 69–82.
MILLNER, R., C. WHITING, M. GREER WALKER, and P. WITTHAMES. 1991. Growth increment, condition and fecundity in sole (Solea solea (L.)) from the North Sea and Eastern English Channel. Neth. J. Sea Res., 27: 433–439. doi:10.1016/0077-7579(91)90044-2
MORGAN, M. J. 2004. The relationship between fish condition and the probability of being mature in American plaice (Hippoglossoides platessoides). ICES J. Mar. Sci., 61: 64–70. doi:10.1016/j.icesjms.2003.09.001
MORGAN, M. J., and J. BRATTEY. 2005. Effect of changes in reproductive potential on perceived productivity of three Northwest Atlantic cod (Gadus morhua) stocks. ICES J. Mar. Sci., 62: 65–74. doi:10.1016/j.icesjms.2004.10.003
MORGAN, M. J., and E. B. COLBOURNE. 1999. Variation in maturity at age and size in three populations of American plaice. ICES J. Mar. Sci., 56: 673–688. doi:10.1006/jmsc.1999.0487
MORGAN, M. J., and G. R. LILLY. 2006. The impact of condition on reproduction in Flemish Cap cod. J. Northw. Alt. Fish. Sci., 37: 81–86. doi:10.2960/J.v37.m560
MORGAN, M. J., and R. M. RIDEOUT. MS 2005. A preliminary examination of spawning suppression in relation to recruitment in NAFO SubDiv. 3Ps cod (Gadus morhua). DFO Can. Sci. Advis. Sec. Res. Doc., 2005/085.
MURAWSKI, S. A., P. J. RAGO, and E. A. TRIPPEL. 2001. Impacts of demographic variation in spawning characteristics on reference points for fishery management. ICES J. Mar. Sci., 40: 1002–1014. doi:10.1006/jmsc.2001.1097
MURUA, H., S. CERVIÑO, and A. VÁZQUEZ. MS 2006. A survey-based assessment of cod in Division 3M. NAFO SCR Doc., No. 32, Serial No. N5253, 10 p.
NEEDLE, C. L. 2002. Recruitment models: diagnosis and prognosis. Rev. Fish Biol. Fish., 11: 95–111. doi:10.1023/A:1015208017674
O’BRIEN, L., N. SHEPHERD, and L. COL. MS 2006. Assessment of the Georges Bank Atlantic cod stock for 2005. Northe. Fish. Cen. Ref. Doc., 06/10, 158 p.
O’FARRELL, M. R., and L. W. BOTSFORD. 2006. The fisheries management implications of maternal-age-dependent larval survival. Can. J. Fish. Aquat. Sci., 63: 2249–2258. doi:10.1139/F06-130
OGANESYAN, S. A. MS 1993. Periodicity of the Barents Sea cod reproduction. ICES CM 1993/G:64, 11 p.
OLSEN, E. M., G. R. LILLY, M. HEINO, M. J. MORGAN, J. BRATTEY, and U. DIECKMANN. 2005. Assessing changes in age and size at maturation in collapsing cod populations. Can. J. Fish. Aquat. Sci., 62: 811–823. doi:10.1139/f05-065
PITT, T. K. 1975. Changes in abundance and certain biological characteristics of Grand Bank American plaice, Hippoglossoides platessoides. J. Fish. Res. Board Can., 32: 1383–1398.
PINHORN, A. T., 1984. Temporal and spatial variation in fecundity of Atlantic cod (Gadus morhua) in Newfoundland waters. J. Northw. Atl. Fish. Sci., 5: 161–170.
PÖRTNER, H. O., B. BERDAL, R. BLUST, O. BRIX, A. COLOSIMO, B. DE WACHTER, A. GIULIANI, T. JOHANSEN, T. FISCHER, R. KNUST, G. LANNIG, G. NAEVDAL, A. NEDENES, G. NYHAMMER, F. J. SARTORIS, I. SERENDERO, P. SIRABELLA, S. THORKILDSEN, and M. ZAKHARTSEV. 2001. Climate induced temperature effects on growth performance, fecundity and recruitment in marine fish: developing a hypothesis for cause and effect relationships in Atlantic cod (Gadus morhua) and common eelpout (Zoarces viviparus). Cont. Shelf Res., 21: 1975–1997. doi:10.1016/S0278-4343(01)00038-3
POWER, M., J. B. DEMPSON, J. D. REIST, C. J. SCHWARZ, and G. POWER. 2005. Latitudinal variation in fecundity among Arctic char populations in eastern North America. J. Fish Biol., 67: 255–273. doi:10.1111/j.0022-1112.2005.00734.x
RICKER, W. E. 1954. Stock and recruitment. J. Fish. Res. Board Can., 11: 559–623.
RIDEOUT, R. M., M. P. M. BURTON, and G. A. ROSE. 2000. Observations on mass atresia and skipped spawning in northern Atlantic cod, from Smith Sound, Newfoundland. J. Fish Biol., 57: 1429–1440. doi:10.1111/j.1095-8649.2000.tb02222.x
RIDEOUT, R. M., and M. J. MORGAN. 2007. Major changes in fecundity and the effect on population egg production for three species of Northwest Atlantic flatfish. J. Fish Biol., 70: 1759–1779.
RIDEOUT, R. M., M. J. MORGAN, and G. R. LILLY. 2006 Variation in the frequency of suppressed reproduction in Atlantic cod (Gadus morhua) off Newfoundland and Labrador. ICES J. Mar. Sci., 63: 1101–1110.
RIDEOUT, R. M., and G. A. ROSE. 2006. Suppression of reproduction in Atlantic cod Gadus morhua. Mar. Ecol. Prog. Ser., 320: 267–277. doi:10.3354/meps320267
RIDEOUT R. M., G. A. ROSE, and M. P. M BURTON. 2005a. Skipped spawning in female iteroparous fishes. Fish Fish., 6: 50–72. doi:10.1111/j.1467-2679.2005.00174.x
RIDEOUT, R. M., E. A. TRIPPEL, and M. K. LITVAK. 2005b. Effects of egg size, food supply and spawning time on early life history success of haddock Melanogrammus aeglefinus. Mar. Ecol. Prog. Ser., 285: 169–180. doi:10.3354/meps285169
RIJNSDORP, A. D. 1989. Maturation of male and female North Sea plaice (Pleuronectes platessa L.). J. Cons. Perm. Inter. Explor. Mer, 46: 35–51.
1990. The mechanism of energy allocation over reproduction and somatic growth in female North Sea plaice, Pleuronectes platessa L. Neth. J. Sea Res., 25: 279–290. doi:10.1016/0077-7579(90)90027-E
1991. Changes in fecundity of female North Sea plaice (Pleuronectes platessa L.) between three periods since 1900. ICES J. Mar. Sci., 48: 253–280. doi:10.1093/icesjms/48.3.253
1993. Fisheries as a large-scale experiment on life-history evolution: disentangling phenotypic and genetic effects in changes in maturation and reproduction of North Sea plaice, Pleuronectes platessa L. Oecologia, 96: 391–401. doi:10.1007/BF00317510
1994. Population-regulating processes during the adult phase in flatfish. Neth. J. Sea Res., 32: 307–223. doi:10.1016/0077-7579(94)90041-8
RIJNSDORP, A. D., N. DAAN, F. A. VAN BEEK, and H. J. L. HEESEN. 1991. Reproductive variability in North Sea plaice, sole and cod. J. Cons. int. Explor. Mer., 47: 352–375.
ROFF, D. A. 1992. The evolution of life histories: theory and analysis. Chapman and Hall, New York, 535 p.
SABORIDO-REY, F., and S. JUNQUERA. 1999. Spawning biomass variation in Atlantic cod (Gadus morhua) in Flemish Cap in relation to changes in growth and maturation. J. Northw. Atl. Fish. Sci., 25: 83–90. doi:10.2960/J.v25.a7
SANDSTRÖM, O., E. NEUMAN, and G. THORESSON. 1995. Effects of temperature on life history variables in perch. J. Fish Biol., 47: 652–670. doi:10.1111/j.1095-8649.1995.tb01932.x
SCOTT, B. E., G. MARTEINSDOTTIR, G. A. BEGG, P. J. WRIGHT, and O. S. KJESBU. 2006. Effects of population size/age structure, condition and temporal dynamics of spawning on reproductive output in Atlantic cod (Gadus morhua). Ecol. Model., 191: 383–415. doi:10.1016/j.ecolmodel.2005.05.015
SOLEMDAL, P., Ø. BERGH, N. FINN, H. J. FYHN, O. GRAHL-NIELSON, O. HOMME, O. S. KJESBU, E. KJORSVIK, J. OPSTAD, and A. B. SKIFTSVIK. MS. 1992. The effects of maternal status of Arcto-Norwegian cod on egg quality and vitality of early larvae. II. Preliminary results of the experiment in 1992. ICES CM1992/S:79.
STACEY, N. E. 1984. Control of the timing of ovulation by exogenous and endogenous factors. In: Fish Reproduction: Strategies and Tactics. G. W. Potts and R. J. Wootton (eds.). Academic Press, London, p. 207–222.
STACEY, N. E., and P. W. SORENSEN. 1991. Function and evolution of fish hormonal pheromones. In: The Biochemistry and Molecular Biology of Fishes, Vol. 1. P. W. Hochachka and T. P. Mommsen (eds.). Elsevier, Amsterdam, p. 109–135.
STEARNS, S. C. 1992. The evolution of life history strategies. Oxford University Press, Oxford, 249 p.
SPENCER, P., D. HANSELMAN, and M. DORN. 2007. The effect of maternal age of spawning on estimation of Fmsy for Alaska Pacific ocean perch. In: Biology, assessment, and management of north Pacific rockfishes, J. Heifetz, J. DiCosmo, A. J. Gharretti, M. S. Love, V. M. O’Connell and R. D. Stanley (eds.). Alaska Sea Grant, Univ. of Alaska, Fairbanks, p. 513–533.
SWINGLE, H. S. 1954. A repressive factor controlling reproduction in fishes. In: Proceedings of the Eighth Pacific Congress of the Pacific Science Association held at the University of the Philippines. Pacific Science Association, Quezon City, p. 865–871.
THORSEN, A., and O. S. KJESBU. 2001. A rapid method for estimation of oocyte size and potential fecundity in Atlantic cod using a computer-aided particle analysis system. J. Sea Res., 46: 295–308. doi:10.1016/S1385-1101(01)00090-9
THORSEN, A., C. T. MARSHALL, and O. S. KJESBU. 2006. Comparison of various potential fecundity models for north-east Arctic cod Gadus morhua, L. using oocyte diameter as a standardizing factor. J. Fish Biol., 69: 1709–1730. doi:10.1111/j.1095-8649.2006.01239.x
TOMKIEWICZ, J., M. J. MORGAN, J. BURNETT, and F. SABORIDO-REY. 2003. Available information for estimating reproductive potential of Northwest Atlantic groundfish stocks. J. Northw. Atl. Fish. Sci., 33: 1–21. doi:10.2960/J.v33.a1
TRIPPEL, E. A. 1995. Age at maturity as a stress indicator in fisheries. Bioscience, 45: 759–771. doi:10.2307/1312628
1998. Egg size and viability and seasonal offspring production of you Atlantic cod. Trans. Amer. Fish. Soc., 127: 339–359. doi:10.1577/1548-8659(1998)127<0339:ESAVAS>2.0.CO;2
TRIPPEL, E. A., and H. H. HARVEY. 1990. Ovarian atresia and sex ratio imbalance in white sucker, Catostomus commersoni. J. Fish Biol., 36: 231–239. doi:10.1111/j.1095-8649.1990.tb05598.x
VALLIN, L., and A. NISSLING. 2000. Maternal effects on egg size and egg buoyancy of Baltic cod, Gadus morhua. implications for stock structure effects on recruitment. Fish. Res., 49: 21–37. doi:10.1016/S0165-7836(00)00194-6
WALSH, S. J. 1994. Life history traits and spawning characteristics in populations of long rough dab (American plaice) Hippoglossoides platessoides (Fabricius) in the north Atlantic. Neth. J. Sea Res., 32: 241–254. doi:10.1016/0077-7579(94)90002-7
WALTERS, C. J., and D. LUDWIG. 1981. Effects of measurement errors on the assessment of stock-recruitment relationships. Can. J. Fish. Aquat. Sci., 38: 704–710.
WRIGHT, P. J. MS 2005. Temporal and spatial variation in reproductive investment of haddock in the North Sea. ICES CM 2005/Q:07, 24 p.
YONEDA, M., and P. J. WRIGHT. 2004. Temporal and spatial variation in reproductive investment of Atlantic cod Gadus morhua in the northern North Sea and Scottish west coast. Mar. Ecol. Prog. Ser., 276: 237–248. doi:10.3354/meps276237
|