Introduction
Hogfish (Lachnolaimus maximus) are large, conspicuous residents of coral reefs in the western North Atlantic Ocean, Gulf of Mexico, and Caribbean Sea (Claro et al., 1989; Westneat, 2002; McBride and Richardson, 2007). Like many other wrasses (Labridae), hogfish are monandric post-maturational protogynous hermaphrodites (McBride and Johnson, 2007). They support a modest commercial and recreational fishery in the southeastern United States, especially in Florida, where about 70% of the U.S. commercial hogfish catch was landed during 2000–2004 (NOAA, 2008). Hogfish are commonly harvested by spear, occasionally landed in line fisheries, and widely considered an excellent food fish (McClane, 1977; McBride and Murphy, 2003).
Hogfish in the Florida Keys show evidence of growth overfishing (McBride and Murphy, 2003), size-selective fishing mortality (McBride and Richardson, 2007), and an overfished condition (Ault et al., 2005). Management of this population currently includes a minimum size limit of 305 mm (12 inches) fork length (FL). Such a minimum size limit could lead to recruitment overfishing in regions where fishing rates are high because: (1) the size limit is set at the smallest size that females were observed to change sex (Davis, MS 1976; McBride and Richardson, 2007), (2) hogfish mate in harems (Colin, 1982), and (3) and sex change requires several months to complete (McBride and Johnson, 2007). Therefore, it is likely that harvesting a male controlling a harem will disrupt the spawning activity and possibly the subsequent spawning success of the females in that harem as well, at least temporarily.
Attempts at producing a biomass-based stock assessment of hogfish have been confounded because most hogfish are harvested by recreational divers using spears and there is no fishery-dependent survey that targets this fishing sector (Kingsley, MS 2004). Although there are ongoing fishery-independent surveys of reef fish in the Florida Keys (e.g., Ault et al., 2005), the condition of hogfish appears much worse in the Florida Keys than elsewhere in Florida (McBride and Murphy, 2003; McBride and Richardson, 2007). In such cases of known data limitations and spatially significant variance in population characteristics, it has been argued that simple, local assessment approaches have the advantage over more sophisticated models that are applied over an entire stock complex (Tuckey et al., 2007). In fact, simple assessment methods, such as a yield-per-recruit model, predict that an increase of the hogfish minimum size limit would markedly improve hogfish yield in the Florida Keys and yield would improve slightly in the eastern Gulf of Mexico (McBride and Murphy, 2003). Increasing the minimum size limit is a common management option to improve yield and long-term sustainability of a fish stock, particularly when trophy fish are sought (Griffiths et al., 2006); nonetheless, no increase has been implemented with hogfish in response to these recent assessment results.
Extension of this per-recruit approach to consider spawner biomass or egg production has not been possible heretofore because of a lack of basic reproductive data for hogfish. Otherwise, such stock assessment models are well established (Prager et al., 1987; Gabriel et al., 1989; Boreman, 1997) and can be applied to hermaphrodites with appropriate modifications (Shepherd and Idoine, 1993). In addition to data on size and age structure of a population, a spawner biomass model requires a maturity ogive for each sex, and the egg production model requires estimates of age-specific annual fecundity as well. Although difficult to obtain, such data can be very useful in terms of management, either in the most basic scenario, such as to set a minimum size limit to allow spawners to reproduce once before harvest (Richards and Rago, 1999), or to consider more sophisticated management options.
In this study, we measured survival, size and age at maturity, as well as spawning frequency and batch fecundity of hogfish in two Florida regions. Two of these traits (survival and fecundity) varied between regions, and the ramifications of such differences were explored further using a spawning biomass-per-recruit (SSB/R) model and an egg-per-recruit model (i.e., lifetime fecundity).
Materials and Methods
Hogfish were collected from November 1995 to April 2001 in two geographic regions of Florida’s coast: the eastern Gulf of Mexico and the Florida Keys, referred to herein as the eastern gulf and south Florida, respectively (Fig. 1). Specimens were collected from both regions in all months of the year. Collections were made with a variety of gear types (in decreasing order of number of fish collected): spear, trawl, trap, and hook and line (McBride, MS 2001). When collections were taken from Florida’s fishery, fish had to be larger than the legal minimum size limit (305 mm FL). Fish were also collected independently of the fishery, which resulted in a broader size range. Additional details of field collecting, ageing fish, and classification of sexuality are reported in McBride and Richardson (2007) and McBride and Johnson (2007).
Fish length is reported as fork length (FL) to the nearest mm, and weight is reported to the nearest 1 g (whole body, BW) or 0.1 g (gonad, GW). Gonadosomatic indices (GSIs) were calculated as GSI = 100 GW / (BW – GW) (McBride and Johnson, 2007).
Age determination used a validated sectioned-otolith method (McBride and Richardson, 2007). Age assignment used a 1 January hatch date because hogfish are winter spawners, and age values were adjusted by the number of months beyond January when each fish was collected (e.g., a 5-year old fish caught in June was assigned as 5.42 years old).
Growth and survival rates were estimated using age-based methods. Data for size at age were fit to a von Bertalanffy growth model:
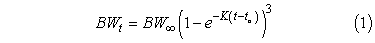
where BWt is the average total body weight (g) at age t, BW∞ is the asymptotic average weight, K is the Brody growth coefficient, t is the age in years (to the nearest month; see above), and to is the predicted age at which the average fish weight is zero (Campana and Jones, 1992). Model fitting used SAS software (PROC NLIN; Freund and Littell, 1991).
Annual survival estimates of female hogfish were calculated using the estimator:
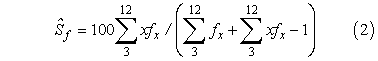
where Ŝf is the percent annual survival of females; x is the coded age-class from the initial age at full recruitment (age 3 = coded age-class 0) to the oldest female age-class observed in both sampling regions (age 12); and fx is the number of females per age-class x (Robson and Chapman, 1961). This annual survival estimate was calculated using only spear collections because most hogfish (1 050 of 1 465 aged fish) were collected by spearing. It was also believed that spearing presented no major gear selectivity issues for legal fish (i.e., fish > age 2), whereas trap and trawl collections were confounded by gear selectivity problems. Annual survival for males and females, Ŝf+m, was also calculated using spear collections and the same equation but with fx representing the number of male and female fish combined.
This ‘cross-sectional’ type of survival analysis assumes equilibrium conditions and is considered reasonable here because many years of collections were pooled together, which dampens out recruitment variation between years. Mortality actually varies within the south Florida region (McBride and Richardson, 2007), but, herein, we have chosen to average out this variation to simplify the presentation; if this variation was included, the differences between regions would be greater but it would not result in a different conclusion regarding hogfish (i.e., that effects of fishing are most evident in south Florida).
The sex of 1 662 fish (1 191 which were aged) was classified based on histological preparations of gonads. A detailed description of hogfish sexual and reproductive development was presented in McBride and Johnson (2007). Herein, only three reproductive stanzas are recognized for each sex: (1) immature, (2) inactive (but mature), and (3) active (and also mature). Immature fish had no indications of spawning readiness or past spawning as that sex: the most advanced oocyte stage of immature females was perinucleolar, whereas regressing oocytes still dominated the ovo-testes of immature males. Inactive, mature fish were regressing or in the early stages of seasonal recrudescence, but did not show clear spawning readiness: the most advanced oocyte stage of inactive females was cortical alveolar, whereas spermatogonia or spermatocytes dominated the seminiferous tissue of inactive males. Active fish were ready to or were actively spawning: vitellogenic oocytes or oocytes in final maturation defined active females, whereas active males had significant amounts of spermatozoa in discontinuous lobular lumen (spermiation) or in sinuses of the tunica.
Other reproductive traits were calculated using these histological data. Size and age at maturity were determined using the binary logit model:
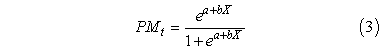
where PMt is the probability of maturity at a particular age or length class, a and b are constants, and X is either length or age. Size or age at 50% maturity =|a⁄b|. Model structure and fitting followed Allison (1999; PROC LOGISTIC). Spawning frequency was estimated by the “hydrated oocyte” as well as the “post-ovulatory follicle” (POF) methods of Hunter and Macewicz (MS 1985). Hydrated oocytes and POFs appeared in hogfish as has been reported for other species (e.g., Brown-Peterson et al., 2000; McBride et al., 2002; McBride and Thurman, 2003), which led us to assume that hydration occurred during the afternoon and that POFs were less than 24 hours old if the thecal and granulosa cell layers were only partially collapsed and still distinct. These assumptions were consistent with the fact that estimates of spawning frequency within each region were not significantly different when using either method (see Results). The proportional occurrence of either histological feature (hydrated oocytes or POFs) was a measure of the average daily female spawning frequency. Separate estimates were made for each month and region and summed to calculate the average number of spawning days per year.
Batch fecundity was estimated gravimetrically (Hunter et al., MS 1985). Three subsamples of hydraulically separated oocytes were weighed to the nearest 0.001g and the numbers of hydrated oocytes were counted (Lowerre-Barbieri and Barbieri, 1993). The average number of oocytes per gram was expanded by the total ovary weight to estimate the total number of eggs spawned per event. Batch fecundity was modeled as:
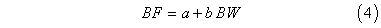
where BF is the number, in thousands, of hydrated eggs per spawning event; a is the y-intercept; b is the regression slope; and BW is the whole body weight (g). The full ANCOVA model for both regions was examined using SAS software (PROC GLM; Freund and Littell, 1991; Littell et al., 1991).
Spawning stock biomass-per-recruit was calculated for each region as:
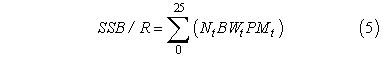
where t is the age-class, set at the middle of the calendar year; Nt is the number of fish per age-class t (No = 1.0 and see below); BWt is calculated for both regions using eq. 1 and the eastern gulf parameters because of evidence of size-selective mortality in south Florida (McBride and Richardson, 2007); PMt is calculated with eq. 3; and t = age, in years (Murawski et al., 2001). Hogfish recruit to the fishery at 305 mm FL (i.e., the minimum size limit), at age 3. Thus, proportional survivorship used in the SSB/R model was applied in two stages. Initially, Ŝf or Ŝf+m was 86%, a value equivalent to an instantaneous mortality rate (M) of 0.15 per year, as proposed by McBride and Murphy (2003). After age 3, this annual survival decreased to reflect fishing mortality as well (Table 1).
Hogfish exhibit asynchronous oocyte development (Claro et al., 1989; McBride and Johnson, 2007). Therefore, lifetime fecundity was estimated as:

where EGGS is expected lifetime number of eggs produced per recruit; Nt and PMt are the same as used for the SSB/R model (eq. 5); BFt is the average region-specific batch fecundity (adjusted by weight using eq. 4 for each age-class by the growth model using eq. 1); and SF is the annual spawning frequency (a fixed value for both regions; see Results).
Biomass and egg production models were also run for a virtual, unfished population (i.e., where survival equaled only the natural mortality rate for all age-classes, estimated as M = 0.15 per year (McBride and Murphy, 2003)). Parameter values for the eastern gulf were entered into the unfished, virtual model, because there was less evidence of fishing effects for this region compared to south Florida (See also, McBride and Richardson, 2007).
Results
Life History by Sampling Region
Some hogfish life history characteristics differed between the eastern gulf and south Florida regions. The mean weight at age began to diverge after age 3, the age at full recruitment to the fishery (Fig. 2). Growth modeling for the eastern gulf predicted a W∞ of 9.48 kg (Table1). The model failed to converge for the south Florida data, but the maximum mean weight at age observed in South Florida was only 3.7 kg, roughly a third of the asymptotic average weight for the eastern gulf.
Annual survival of females was significantly higher in the eastern gulf (Ŝf = 75.1) than in south Florida (60.8) (ts = 4.8, df = 297, P < 0.0001) (Table 1, Fig. 3). Annual survival of females and males combined was also significantly higher in the eastern gulf (Ŝ = 76.9) than in south Florida (69.7) (ts = 5.7, df = 678, P < 0.0001).
Female size at 50% maturity was significantly larger (192.7 vs. 169.0 mm FL; χ2 = 38.7, df = 1, P < 0.0001) in south Florida (Table 1, Fig. 4). Female age at 50% maturity was also significantly older (1.6 vs. 1.1 years; χ2 = 33.9, df = 1, P < 0.0001) in south Florida. Although male size at 50% maturation was not significantly different between regions (416 vs. 426 mm FL; χ2 = 0.34, df = 1, P = 0.34), males matured about six months later in south Florida compared to the eastern gulf (7.0 vs. 6.5 years; χ2 = 19.2, df = 1, P < 0.0001).
Hogfish are principally winter spawners, although some spawning in Florida was evident nearly year round (Fig. 5A, B). Spawning frequency was only slightly but consistently higher in the eastern gulf as calculated by two methods. Female hogfish spawned an estimated average of 72 days a year in the eastern gulf vs. 68 days in south Florida as calculated by the hydrated oocyte method; they spawned an estimated average of 84 days per year in the eastern gulf vs. 64 days in south Florida as calculated by the POF method. There were no significant differences in monthly spawning frequencies between regions (using the same method) or between methods for the same region (Wilcoxon signed ranked test of monthly spawning frequencies, P > 0.05). Therefore all data were pooled resulting in an average female spawning frequency of 72 days per year. Although spawning rates did not differ between regions, spawning seasonality in the eastern gulf lagged 1–2 months behind south Florida (Fig. 5A, B).
The relative gonad size, as measured by GSI, was consistently larger in the eastern gulf than in south Florida, for both males and females (Fig. 5C, D), and female batch fecundity estimates were higher in the eastern gulf (Table 1, Fig. 6). Batch fecundity was significantly and linearly related to female body weight for the eastern gulf (ts = 2.78, df = 1, P = 0.01), but not for south Florida (ts = 1.11, df = 1, P = 0.30). Presumably this regression model is significant for both regions, but the lack of significance in south Florida could be a result of low sample size. A full ANCOVA model showed that females from the eastern gulf produced larger batches of eggs than females in south Florida, after accounting for body weight (interaction of slopes was not significant Fs = 0.60, df = 1, P = 0.44, but the intercepts were significantly different Fs = 6.0, df = 1, P = 0.02).
Per-recruit Models
Female SSB/R was much higher in the eastern gulf than in south Florida (Table 2). The higher mortality rates measured in south Florida had a dramatic impact on female spawner biomass after age 3, when hogfish recruit to the fishery (Fig. 7A). The female SSB/R in the eastern gulf was 38.3% of the expected SSB/R of an unfished population, which was well over twice that of the female SSB/R in south Florida (16.2%).
Male SSB/R was also much higher in the eastern gulf than in south Florida (Table 2). Not all individuals were male once hogfish recruit to the fishery, so male spawning biomass did not peak until age 9 (Fig. 7B), much later than for females. Absolute values of male SSB/R were lower compared to female SSB/R values within each region, because there was a several year difference in the age at 50% maturation between sexes. Nonetheless, relative to an unfished population, region-specific male SSB/R values were similar to female SSB/R values: 35.8% for the eastern gulf and 16.7% for south Florida.
The effects of significantly lower survival, later maturation, and lower batch fecundity for south Florida hogfish lead to notably reduced annual fecundity in all age-classes (Fig. 7C). The lifetime expected egg production between the eastern gulf and south Florida was nearly a 5-fold difference: 9.7 vs. 2.1 million eggs per recruit, respectively (Table 2). Relative to an unfished population, egg production in the eastern gulf was still relatively high (38.3%) but it was only 8.3% in south Florida.
Discussion
Life History by Sampling Region
Although weight at age varied notably for hogfish between the eastern gulf and south Florida, we believe the significant differences in survivorship between the regions are affecting size at age, rather than growth rate differences per se. The rationale for this conclusion is explored more completely by McBride and Richardson (2007), where it is noted that the sizes at age of pre-recruits (< age 3) are the same between regions and differences arise only after recruitment to the fishery. McBride and Richardson (2007) also discuss Claro et al.’s (1989) study, which shows a high maximum size among Cuban hogfish, and therefore precludes a simple interpretation of a latitudinal life history pattern of size at age (Conover, 1992).
Herein, the survival rate estimate of post-recruit females, which treats sex change as equivalent to death of a female, was higher in the eastern gulf than for south Florida. McBride and Richardson (2007) also reported higher survival of post-recruitment fish, male and female, in the eastern gulf. In fact, in south Florida, fishing mortality rates are high enough to exhibit Lee’s phenomenon (Francis, 1990), where the faster growing fish are measurably selected out of the population once they reach the minimum size limit (305 mm FL).
The significant difference in age at maturation, occurring about a half a year later for hogfish of both sexes in south Florida, was unexpected. In a simple mating system, and assuming that a maturation schedule is a heritable trait, then it would be predicted that the population with higher mortality would mature at a younger age (Leggett and Carscadden, 1978; Jørgensen, 1990; Trippel, 1995). But, in terms of female hogfish, a difference of less than one year in 50% age at maturation may not consistently affect an individual’s fitness because females in both regions have at least one full season to reproduce before they recruit to the fishery. Or, in terms of male hogfish, it is not necessary to conclude that males should mature at an earlier age when mortality is higher, because hogfish are post-maturational (McBride and Johnson, 2007); therefore males were reproductively active as females before changing sex. And, in terms of the hogfish mating system, regional differences in age at maturation may be confounded if sex ratios within harems (not observed in this study) vary significantly between regions.
Thus, the regional differences in maturation ogives, although statistically different, were difficult to evaluate because of the hermaphroditic sexuality and harem mating system of hogfish. Nonetheless, these new ogives have practical use for evaluating regulatory options for single-species management of hogfish (Pearset al., 2006). In particular, the size of 50% male maturation, approximately 415–425 mm (16.3–16.7 inches) FL, is well above the current minimum size limit. Evidently, to reduce disruption to spawning harems and avoid recruitment overfishing, the minimum size limit should be increased.
Monthly spawning frequency estimates were not significantly different between the two regions, but batch fecundity estimates were significantly higher in the eastern gulf versus south Florida. We speculate here that the higher fishing pressure in south Florida is affecting the allocation of energy between somatic and gonadal growth, with the effect that females in south Florida continue spawning at the same frequency as in the eastern gulf, but with smaller egg batches. Two possibilities were likely; either females produce fewer eggs if smaller males are present, or females are allocating less energy to current fecundity to maintain an option to be (somatically) bigger and therefore more likely to change sex to a male at the end of the spawning season (or both possibilities are realized). If so, then one testable prediction of an increase in the minimum size limit is a corresponding increase in batch fecundity for hogfish in south Florida.
These postulations demonstrate the need to conduct more process-oriented observations of spawning and mating behavior of the entire harem – males and females – and how these affect female life history traits, which in turn affect egg production. For example, high and heteroscedastic variance of labrid fecundity vs. size relationship have been observed elsewhere and may reflect important biological traits. Robertson et al. (1999) pointed out that on days when relatively more Thallassoma bifasciatum spawned on a certain reef their clutches were slightly larger. It may also be possible that energetic constraints are affecting egg production in south Florida (e.g., Somarakis et al., 2006), but we have no information that food supply – specifically the invertebrates that hogfish feed on – is limiting in this system.
Per-recruit Models
Hogfish SSB/R peaked at an earlier age for females, because, as protogynous hermaphrodites, females mature at a much younger age than males. Also, the total SSB/R was notably higher for females than males in each region, because the later-maturing males experience higher cumulative mortality on average. SSB/R models are commonly used to evaluate a population’s response to the combined effects of mortality and size or age at recruitment to a fishery (Gabriel et al., 1989; Shepherd and Idoine, 1993). Relative to an unfished population the SSB/R was similar for each sex whether calculated for the eastern gulf (36–38%) or south Florida (16–17%). A minimum threshold SSB/R of 20 or 30% is generally proposed for fish stocks (Gabriel et al., 1989; Goodyear, 1993), for which the eastern gulf population exceeded but which the south Florida population did not.
The egg production model demonstrated, however, that when SSB/R models treat all females equally they can overstate the resiliency of populations to size-selective fishing. In this regard, the eastern gulf maintained relatively good egg production levels, 38% of the unfished maximum, but south Florida production was at only 8%. Lower survivorship and lower batch fecundity in south Florida, both associated with higher fishing pressure (McBride and Murphy, 2003; McBride and Richardson, 2007), contributed to significantly lower egg production.
Such patterns are not unexpected, but the data are not typically available. Murawski et al. (2001) synthesized the effects of age and size truncation on many different reproductive parameters for Atlantic cod (Gadus morhua), a particularly well-studied marine species. They found that once larger and older fish are selectively harvested out of the population the demographics shift to younger, inexperienced females and the reproductive success of the cohort decreases. This happens in many ways including shorter spawning periods, lower batch fecundities, declining spawning frequencies, and even smaller eggs; in turn, these changes lead to lower fertilization rates, decreased hatching rates, and higher larval mortality. Egg and embryo production increases significantly with maternal age for livebearing fishes as well (Bobko and Berkeley, 2004; Berkeley et al. 2004). These problems – using spawning stock biomass as a proxy for reproductive potential – persist for biomass assessment models (i.e., those with stock-recruit relationships) as well (Marshall et al., 2006).
The per-recruit models developed herein pooled multiple years of collections and assumed equilibrium conditions (see Methods). Given this assumption of equilibrium conditions and using a virtual, unfished population as a benchmark, the per-recruit models used here set a common currency for evaluating the effects of regional life history differences on reproductive potential of hogfish. This life history approach is useful as a stock assessment method because fecundity is related to recruitment in many marine fish populations (Rickman et al., 2000).
Alternative assessment methods are available, if spawning behaviors, mating systems, and cohort-specific mortality rates are included in the model. For example, Warner (1984) developed a lifetime fitness model of a reef fish, using the net reproductive rate (Ro) for male and female T. bifasciatum (Labridae). Warner estimated time budgets for reproduction versus feeding only, which he considered the two main behavioral modes for this species. Time spent reproducing decreases time spent feeding, increases expenditures of gametes, and reduces growth rates. When sexual selection is high, as was on the small reefs in Warner’s study, male T. bifasciatum spent more time feeding and grow faster. Faster growth increases their potential for attaining the terminal sexual phases and increasing their fitness. Further behavioral research with hogfish could provide an independent assessment of the tradeoffs of sex change by hogfish, and thereby lead to a better prediction of both evolutionary fitness and fishery benchmarks for protogynous species.
Previous research for hogfish in Florida demonstrated with a yield-per-recruit model that growth overfishing was occurring in south Florida (McBride and Murphy, 2003; Ault et al., 2005). Herein, both SSB/R and an egg-per-recruit models suggest that recruitment overfishing is also a problem, again, in south Florida but not in the eastern gulf. These models could eventually incorporate more detailed or complex parameters, such as cohort- or age-specific mortality, variable growth rates or longevity. Nonetheless, the simplicity of the data herein demonstrate the potential for SSB/R and egg production models to produce different assessment outcomes. Although more research would be insightful regarding the effects of size-selective fishing pressure on hogfish maternal effects or mating systems, the models presented here are a suitable starting point for evaluating alternative management strategies as equivalent options for improving hogfish spawning biomass and egg production in Florida (Prager et al., 1987; Shepherd and Idoine, 1993).
Acknowledgements
Samples were obtained with the assistance of J. Colvocoresses, D. DeMaria, K. DeMaria, T. Dunmire, J. Hunt, M. Johnson, M. Larkin, D. Murie, D. Snodgrass, P. Steele, and J. Styer. This manuscript was improved with comments from A. Collins, J. Colvocoresses, and J. Munyandorero. Funding for sampling and life history research was provided by a Marine Fisheries Initiative (MARFIN, National Oceanic and Atmospheric Administration, NOAA grant no. NA87FF0422). Collections were also provided from sampling programs funded by Florida Saltwater Fishing License sales, the Department of the Interior (U. S. Fish and Wildlife Service, Federal Aid for Sportfish Restoration, Project F-43), and by award (NA36FI0125) from NOAA to the Florida Department of Natural Resources. Administration of this latter award and its study products was subsequently transferred to the Florida Department of Environment Protection and then to the Florida Fish and Wildlife Conservation Commission. The statements, findings, conclusions, and recommendations are those of the authors and do not necessarily reflect the views of the Department of Commerce or NOAA or any of its subagencies. We are grateful for everyone’s help in this study.
References
ALLISON, P. D. 1999. Logistic regression using the SAS System. SAS Institute, Inc., Cary, NC, 288 p.
AULT, J. S., S. G. SMITH, and J. A. BOHNSACK. 2005. Evaluation of average length as an estimator of exploitation status for the Florida coral-reef fish community. ICES J. Mar. Sci., 62: 417–423. doi:10.1016/j.icesjms.2004.12.001
BERKELEY, S. A., M. A. HIXON, R. J. LARSON, and M. S. LOVE. 2004. Fisheries sustainability via protection of age structure and spatial distribution of fish populations. Fisheries, 29: 23–31. doi:10.1577/1548-8446(2004)29[23:FSVPOA]2.0.CO;2
BOBKO, S. J., and S. A. BERKELEY. 2004. Maturity, ovarian cycle, fecundity, and age-specific parturition of black rockfish (Sebastes melanops). Fish. Bull., 102: 418–429.
BOREMAN, J. 1997. Methods for comparing the impacts of pollution and fishing on fish populations. Trans. Amer. Fish. Soc., 126: 506–513. doi:10.1577/1548-8659(1997)126<0506:MFCTIO>2.3.CO;2
BROWN-PETERSON, N. J., R. M. OVERSTREET, J. M. LOTZ, J. S. FRANKS, and K. M. BURNS. 2000. Reproductive biology of cobia, Rachycentron canadum, from coastal waters of the southern United States. Fish. Bull., 99: 15–28.
CAMPANA, S. E., and C. M. JONES. 1992. Analysis of otolith microstructure data. Can. Spec. Publ. Fish. Aquat. Sci., 117: 73–100.
CLARO, R., A. GARCIA-CAGIDE, and R. FERNÁNDEZ DE ALAIZA. 1989. Caracteristicas biológicas del pez perro, Lachnolaimus maximus (Walbaum), en el golfo de Batabanó, Cuba. Revista Investigaciones Marinas, 10: 239–252.
COLIN, P. L. 1982. Spawning and larval development of the hogfish, Lachnolaimus maximus (Pisces: Labridae). Fish. Bull., 80: 853–862.
CONOVER, D. O. 1992. Seasonality and the scheduling of life history at different latitudes. J. Fish Biol., 41(Suppl. B): 161–178. doi:10.1111/j.1095-8649.1992.tb03876.x
DAVIS, J. C. MS 1976. Biology of the hogfish, Lachnolaimus maximus (Walbaum), in the Florida Keys. M. S. Thesis, Univ. Miami, Coral Gables, FL, 86 p.
FRANCIS, R. I. C. C. 1990. Back-calculation of fish length: a critical review. J. Fish Biol., 36: 883–902. doi:10.1111/j.1095-8649.1990.tb05636.x
FREUND, R. J., and R.C. LITTELL 1991. SAS System for Regression. SAS Institute, Inc., Cary, NC, 210 p.
GABRIEL, W. L., M. P. SISSSENWINE, and W. J. OVERHOLTZ. 1989. Anaysis of spawning stock biomass per recruit: an example for Georges Bank haddock. N. Am. J. Fish. Manage., 9: 383–391. doi:10.1577/1548-8675(1989)009<0383:AOSSBP>2.3.CO;2
GOODYEAR, C. P. 1993. Spawning stock biomass per recurit in fisheries management: foundation and current use. Can. Spec. Publ. Fish. Aquat. Sci., 120: 67–81.
GRIFFITHS, S. P., G. C. FRY, and T. D. VAN DER VELDE. 2006. Population dynamics and fishery benefits of a large legal size of a pelagic sportfish, the Talang queenfish, Scomberoides commersonnianus, in northern Australia. Fish. Res., 82: 74–86. doi:10.1016/j.fishres.2006.08.005
HUNTER, J. R., N. C. H. LO, and R. J. H. LEONG. 1985. Batch fecundity in multiple spawning fishes. In: An Egg Production Method for Estimating Spawning Biomass of Pelagic Fish: Application to the Northern Anchovy, Engraulis mordax. R. Lasker (ed.). U.S. Dept. Commerce, NOAA Tech. Rep. NMFS, 36: 67–78.
HUNTER, J. R., and B. J. MACEWICZ. 1985. Measurement of spawning frequency in multiple spawning fishes. In: An Egg Production Method for Estimating Spawning Biomass of Pelagic Fish: Application to the Northern Anchovy, Engraulis mordax. R. Lasker (ed.). U.S. Dept. Commerce, NOAA Tech. Rep. NMFS, 36: 79–94.
JØRGENSEN, T. 1990. Long-term changes in age at sexual maturity of northeast arctic cod (Gadus morhua L.). J. Cons. int. Explor. Mer, 46: 235–248.
KINGSLEY, M. C. S. MS 2004. The hogfish in Florida: Assessment Review and Advisory Report. Southeast Data and Assessment Review. Prepared for the South Atlantic Fishery Management Council, the Gulf of Mexico Fishery Management Council, and the National Marine Fisheries Service., 15 p.http://www.sefsc.noaa.gov/sedar/download/SEDAR6_AR2_Hogfish.pdf?id=DOCUMENT
LEGGETT, W. C., and J. E. CARSCADDEN. 1978. Latitudinal variation in reproductive characteristics of American shad (Alosa sapidissima): Evidence for population specific life history strategies in fish. J. Fish. Res. Board Can., 35: 1469–1478.
LITTELL, R. C., R. J. FREUND, and P. C. SPECTOR. 1991. SAS System for Linear Models. SAS Institute, Inc., Cary, NC, 329 p.
LOWERRE-BARBIERI, S. K., and L. R. BARBIERI. 1993. A new method of oocyte separation and preservation for fish reproductive studies. Fish. Bull., 91: 165–170.
MARSHALL, C. T., C. L. NEEDLE, A. THORSEN, O. S. KJESBU, and N. A. YARAGINA. 2006. Systematic bias in estimates of reproductive potential of an Atlantic cod (Gadus morhua) stock: implications for stock–recruit theory and management. Can. J. Fish. Aquat. Sci., 63: 980–994. doi:10.1139/F05-270
McBRIDE, R. S. MS 2001. Age, growth, and reproduction of hogfish, Lachnolaimus maximus. Final Report for Marine Fisheries Initiative (MARFIN) Program (NOAA Award Number NA87FF0422). Florida Marine Research Institute Report FO723-98-00-F, St. Petersburg, FL. 35 p.
McBRIDE, R. S., and M. R. JOHNSON. 2007. Sexual development and reproductive cycling of hogfish (Labridae: Lachnolaimus maximus), a hermaphroditic reef fish. J. Fish Biol., 71: 1270–1292. doi:10.1111/j.1095-8649.2007.01580.x
McBRIDE, R. S., and M. D. MURPHY. 2003. Current and potential yield per recruit of hogfish, Lachnolaimus maximus, in Florida. Proc. Gulf Caribb. Fish. Inst., 54: 513–525.
McBRIDE, R. S., and A. K. RICHARDSON. 2007. Evidence of size-selective fishing mortality from an age and growth study of hogfish (Labridae: Lachnolaimus maximus), a hermaphroditic reef fish. Bull. Mar. Sci., 80: 401–417.
McBRIDE, R. S., F. J. STENGARD, and B. MAHMOUDI. 2002. Maturation and diel reproductive periodicity of round scad (Carangidae: Decapterus punctatus). Mar. Biol., 140: 713–722. doi:10.1007/s00227-001-0759-4
McBRIDE, R. S., and P. E. THURMAN. 2003. Reproductive biology of Hemiramphus brasiliensis and H. balao (Hemiramphidae): maturation, spawning frequency, and fecundity. Biol. Bull., 204: 57–67. doi:10.2307/1543496
McCLANE, A. J. 1977. The Encyclopedia of Fish Cookery. Holt, Rinehart and Winston, New York. 511 p.
MURAWSKI, S. A., P. J. RAGO, and E. A. TRIPPEL. 2001. Impacts of demographic variation in spawning chacteristics on reference points for fishery management. ICES J. Mar. Sci., 58: 1002–1014. doi:10.1006/jmsc.2001.1097
NOAA. 2008. NOAA/NMFS commercial fisheries data website: http://www.st.nmfs.noaa.gov/st1/commercial/landings/annual_landings.html
PEARS, R. J., J. H. CHOAT, B. D. MAPSTONE, and G. A. BEGG. 2006. Demography of a large grouper, Epinephelus fuscoguttatus, from Australia's Great Barrier Reef: implications for fishery management. Mar. Ecol. Prog. Ser., 307: 259–272. doi:10.3354/meps307259
PRAGER, M. H., J. F. O'BRIEN, and S. B. SAILA. 1987. Using lifetime fecundity to compare management strategies: a case history for striped bass. N. Am. J. Fish. Manage., 7: 403–409. doi:10.1577/1548-8659(1987)7<403:ULFTCM>2.0.CO;2
RICHARDS, R. A., and P. J. RAGO. 1999. A case history of effective fishery management: Chesapeake Bay Striped Bass. N. Am. J. Fish. Manage., 19: 356–373.
RICKMAN, S. J., N. K. DULVY, S. JENNINGS, and J. D. REYNOLDS. 2000. Recruitment variation related to fecundity in marine fishes. Can. J. Fish. Aquat. Sci., 57: 116–124. doi:10.1139/cjfas-57-1-116
ROBERTSON, D. R., S. E. SWEARER, K. KAUFMANN, and E. B. BROTHERS. 1999. Settlement vs. environmental dynamics in a pelagic-spawning reef fish at Caribbean Panama. Ecol. Monogr., 69: 195–218.
ROBSON, D. S., and D. G. CHAPMAN. 1961. Catch curves and mortality rates. Trans. Am. Fish. Soc., 90: 181–189. doi:10.1577/1548-8659(1961)90[181:CCAMR]2.0.CO;2
SHEPHERD, G. R., and J. S. IDOINE. 1993. Length-based analyses of yield and spawning biomass per recruit for black sea bass Centropristis striata, a protogynous hermaphrodite. Fish. Bull., 91: 328–337.
SOMARAKIS, S., K. GANIAS, A. SIAPATIS, C. KOUTSIKOPOULOS, A. MACHIAS, and C. PAPACONSTANTINOU. 2006. Spawning habitat and daily egg production of sardine (Sardina pilchardus) in the eastern Mediterranean. Fish. Oceanogr., 15: 281–292. doi:10.1111/j.1365-2419.2005.00387.x
TRIPPEL, E. A. 1995. Age at maturity as a stress indicator in fisheries. Bioscience, 45: 759–771. doi:10.2307/1312628
TUCKEY, T., N. YOCHUM, J. HOENIG, J. LUCY, and J. CIMINO. 2007. Evaluating localized vs. large-scale management: the example of tautog in Virginia. Fisheries, 32: 21–28.
WARNER, R. R. 1984. Deferred reproduction as a response to sexual selection in a coral reef fish: a test of the life historical consequences. Evolution, 38: 148–162. doi:10.2307/2408554
WESTNEAT, M. W. 2002. Labridae. In: The Living Marine Resources of the Western Central Atlantic. Volume 3: Bony Fishes Part 2 (Opistognathidae to Molidae), Sea turtles and Marine Mammals. K. E. Carpenter (ed.). FAO Species Identification Guide for Fishery Purposes and American Society of Ichthyologists and Herpetologists Special Publication No. 5. Food Agric. Organ., Rome, 1701–1722 p.
|